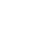The primary goal of treating obesity is to improve health. Currently, about 1 billion people worldwide are obese, and about two-thirds of them are pre-diabetic. Pre-diabetes is characterized by insulin resistance and beta cell dysfunction, leading to a lifetime risk of developing type 2 diabetes of up to 70%.
As a chronic neuroendocrine disease, obesity is a major risk factor for pre-diabetes and type 2 diabetes. The earliest studies looked at whether lifestyle interventions, medications, or weight-loss surgery could prevent or delay the onset of type 2 diabetes. Follow-up studies have focused on restoring normal blood sugar levels, as prediabetes itself carries multiple health risks, including microvascular and macrovascular complications. Drug treatments that directly target obesity and abnormal blood sugar may have unique advantages. Clinically significant sustained weight loss improves insulin sensitivity, while direct action on islets enhances glucose-dependent insulin secretion. The two work together to help restore metabolic balance. Such pharmacological interventions can be used not only to treat obesity, but also to prevent pre-diabetes from progressing to type 2 diabetes, while providing the opportunity to restore normal blood sugar and maximize health benefits.
Tirzepatide is a unique dual receptor agonist capable of simultaneously activating glucose-dependent insulin-stimulating polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptors. It has been approved by the US Food and Drug Administration (FDA), the European Medicines Agency (EMA) and the Chinese Food and Drug Administration for the treatment of obesity and type 2 diabetes. Tilpotide therapy can significantly reduce weight, control blood sugar, and improve other metabolic markers, regardless of whether a patient has type 2 diabetes.
During the first 72 weeks of the SURMOUNT-1 trial, obese patients treated with 15 mg of Tirzepatide lost an average of more than 20 percent of their body weight and reduced their glycosylated hemoglobin levels by 0.51 percent. The secondary endpoint of the study was data on the efficacy and safety of tilpotide for 176 weeks in patients with prediabetes, including its effect on long-term weight management and prevention of progression from prediabetes to type 2 diabetes.
This international, double-blind, randomized, placebo-controlled Phase 3 trial of Tirzepatide in obese patients enrolled obese participants without prediabetes for 72 weeks, compared with 176 weeks for obese participants with prediabetes at baseline. Data on participants without prediabetes at baseline have been reported, and data on patients with prediabetes at baseline are reported.
Participants were randomly assigned to receive 5 mg (247 people), 10 mg (262 people), or 15 mg (253 people) of Tipotide or placebo (270 people) subcutaneously once a week for 176 weeks of treatment followed by a 17-week non-treatment period (drug safety follow-up). The total duration of the trial was 193 weeks. All groups received lifestyle interventions, including regular lifestyle guidance from a dietitian or health care provider that emphasized a healthy and balanced diet, reduced intake of 500 kcal per day, and at least 150 minutes of physical activity per week.
Participants had a mean baseline HbA1c of 5.8%, weight of approximately 236 pounds, mean body mass index (BMI) of 38.8, and waist circumference of 116.5 cm; Of the participants, 35.8 percent had dyslipidemia and 41.2 percent had high blood pressure.
At 176 weeks, nearly all participants receiving Tirzepatide (91 to 95 percent) lost at least 5 percent of their body weight, compared to 25 percent in the placebo group. Participants in the 15 mg telpotide group lost an average of 19.7% of their body weight, compared with 18.7% in the 10 mg group, 12.3% in the 5 mg group, and 1.3% in the placebo group (P<0.001).
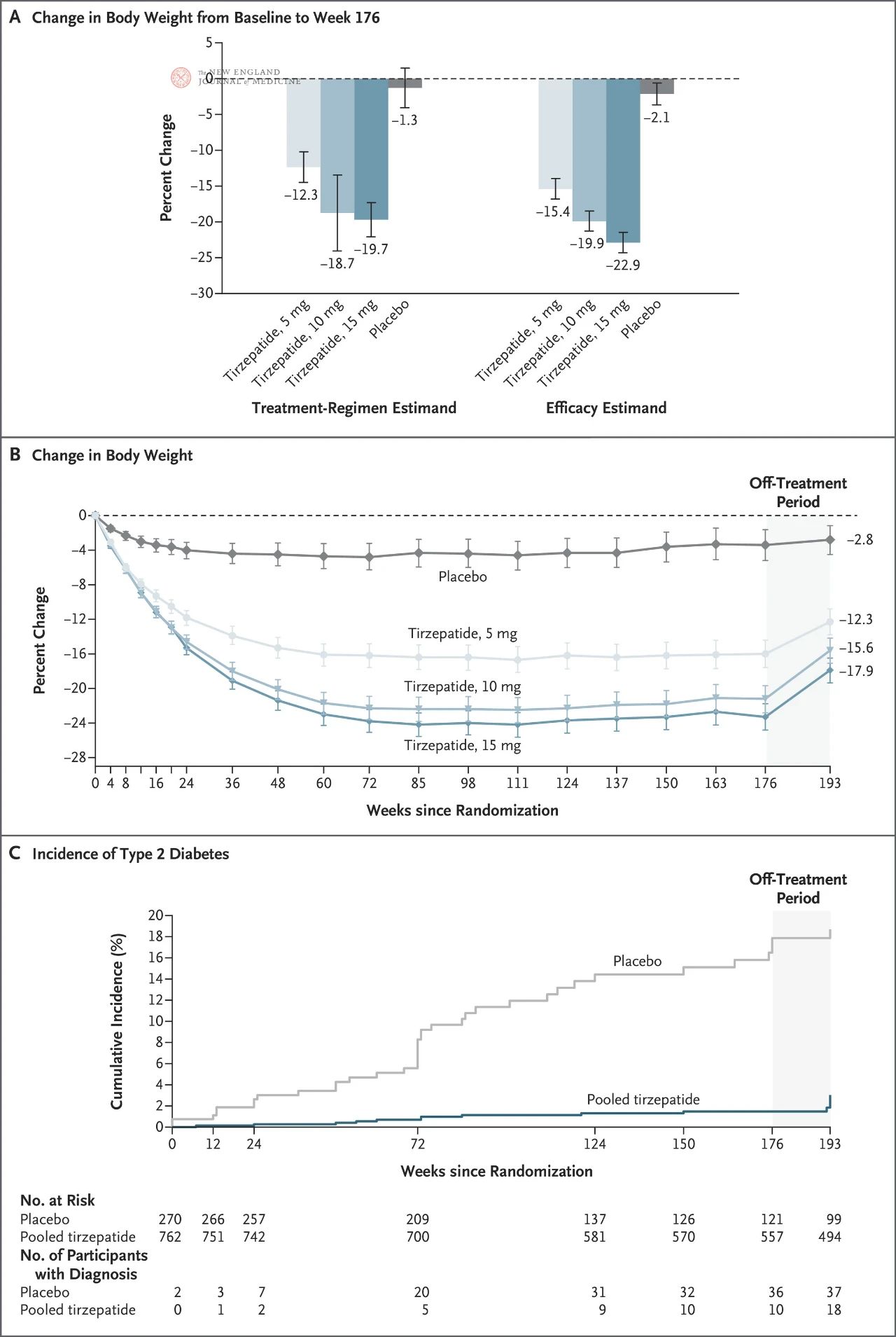
Compared with the placebo group, participants in the tirzepatide group had a 93% lower risk of developing diabetes over 3 years (HR, 0.07; P<0.001). In the tirzepatide group, only 1.3% of participants developed diabetes, compared to 13.3% in the placebo group. Weight loss contributed more than half (55.2%) of the reduction in diabetes risk.
Among patients treated with any dose of tirzepatide, 94.5 percent moved from pre-diabetes to normal blood sugar, compared with 60.4 percent in the placebo group. None of the participants in the 15 mg dose group developed diabetes, and only 2.8 percent still had prediabetes. tirzepatide participants’ absolute HbA1c decreased by an average of 0.5% to 0.65%.
Compared with participants in the placebo group, participants in the tirzepatide group had greater improvements in all aspects of health-related quality of life: physical functioning, physical pain, general perception of health, vitality, social functioning, emotional role functioning, and mental health.
The average reduction in waist circumference in the tirzepatide group was eight times greater than in the placebo group. Patients in the placebo group had an average waist circumference reduction of 1 inch, while participants in the 15 mgtirzepatide group had an average waist circumference reduction of 7.9 inches, those in the 10 mg group had an average waist circumference reduction of 7.2 inches, and those in the 5 mg group had an average waist circumference reduction of 5.1 inches.
Improvements in systolic blood pressure were observed during increased doses of tirzepatide, remained constant throughout treatment, and appeared to be dose-independent. Systolic blood pressure decreased by 0.2 mm Hg in the placebo group and 5.9 to 8.5 mm Hg in the tirzepatide group. Participants in the placebo group experienced a decrease in diastolic blood pressure of 1.9 mmHg, while those in the tirzepatide group experienced a decrease in diastolic blood pressure of 4.2 to 5.9 mmHg. In addition, all participants showed consistent improvements in blood lipid levels, with pooled data for the tirzepatide group showing an average 14 percent increase in HDL and a 32.4 percent decrease in triglycerides, compared with 2.5 percent and 4.2 percent, respectively, in the placebo group.
But 17 weeks after stopping treatment with tirzepatide, at the 193-week follow-up, participants had regained an average of 7 percent of their body weight, and eight more participants in the tirzepatide group had developed type 2 diabetes. Mean HbA1c levels as well as systolic and diastolic blood pressure began to rise toward baseline in participants no longer receiving tirzepatide.
In terms of safety, there was no imbalance in the number of deaths among the treatment groups, and the rates of treatment-related adverse events and serious adverse events were similar among the groups. Serious adverse events occurred in 13.4%, 14.5%, 12.6% and 11.9% of participants in the 15 mg, 10 mg and 5 mg Tirzepatide and placebo groups, respectively. Patients in the tirzepatide group stopped treatment due to adverse events (primarily gastrointestinal side effects) (7.3% to 12.3%) compared to those in the placebo group (5.9%)
Post time: Nov-16-2024