Seasonal epidemics of influenza cause between 290,000 and 650,000 respiratory disease-related deaths worldwide each year. The country is experiencing a serious flu pandemic this winter after the end of the COVID-19 pandemic. Influenza vaccine is the most effective way to prevent influenza, but the traditional influenza vaccine based on chicken embryo culture has some shortcomings, such as immunogenic variation, production limitation and so on.
The advent of recombinant HA protein gene engineering influenza vaccine can solve the defects of traditional chicken embryo vaccine. Currently, the American Advisory Committee on Immunization Practices (ACIP) recommends high-dose recombinant influenza vaccine for adults ≥65 years of age. However, for people under 65 years of age, ACIP does not recommend any age-appropriate influenza vaccine as a priority due to the lack of head-to-head comparisons between different types of vaccines.
The quadrivalent recombinant hemagglutinin (HA) genetically engineered influenza vaccine (RIV4) has been approved for marketing in several countries since 2016 and is currently the mainstream recombinant influenza vaccine in use. RIV4 is produced using a recombinant protein technology platform, which can overcome the shortcomings of traditional inactivated vaccine production limited by the supply of chicken embryos. Moreover, this platform has a shorter production cycle, is more conducive to the timely replacement of candidate vaccine strains, and can avoid adaptive mutations that may occur in the production process of viral strains that may affect the protective effect of finished vaccines. Karen Midthun, then director of the Center for Biologics Review and Research at the U.S. Food and Drug Administration (FDA), commented that “the advent of recombinant influenza vaccines represents a technological advance in the production of influenza vaccines… This provides the potential for faster start-up of vaccine production in the event of an outbreak “[1]. In addition, RIV4 contains three times more hemagglutinin protein than standard dose conventional influenza vaccine, which has stronger immunogenicity [2]. Existing studies have shown that RIV4 is more protective than the standard-dose flu vaccine in older adults, and more complete evidence is needed to compare the two in younger populations.
On December 14, 2023, the New England Journal of Medicine (NEJM) published a Study by Amber Hsiao et al., Kaiser Permanente Vaccine Study Center, KPNC Health System, Oakland, USA. The study is a real-world study that used a population-randomized approach to evaluate the protective effect of RIV4 versus a quadrivalent standard-dose inactivated influenza vaccine (SD-IIV4) in people under 65 years of age during two influenza seasons from 2018 to 2020.
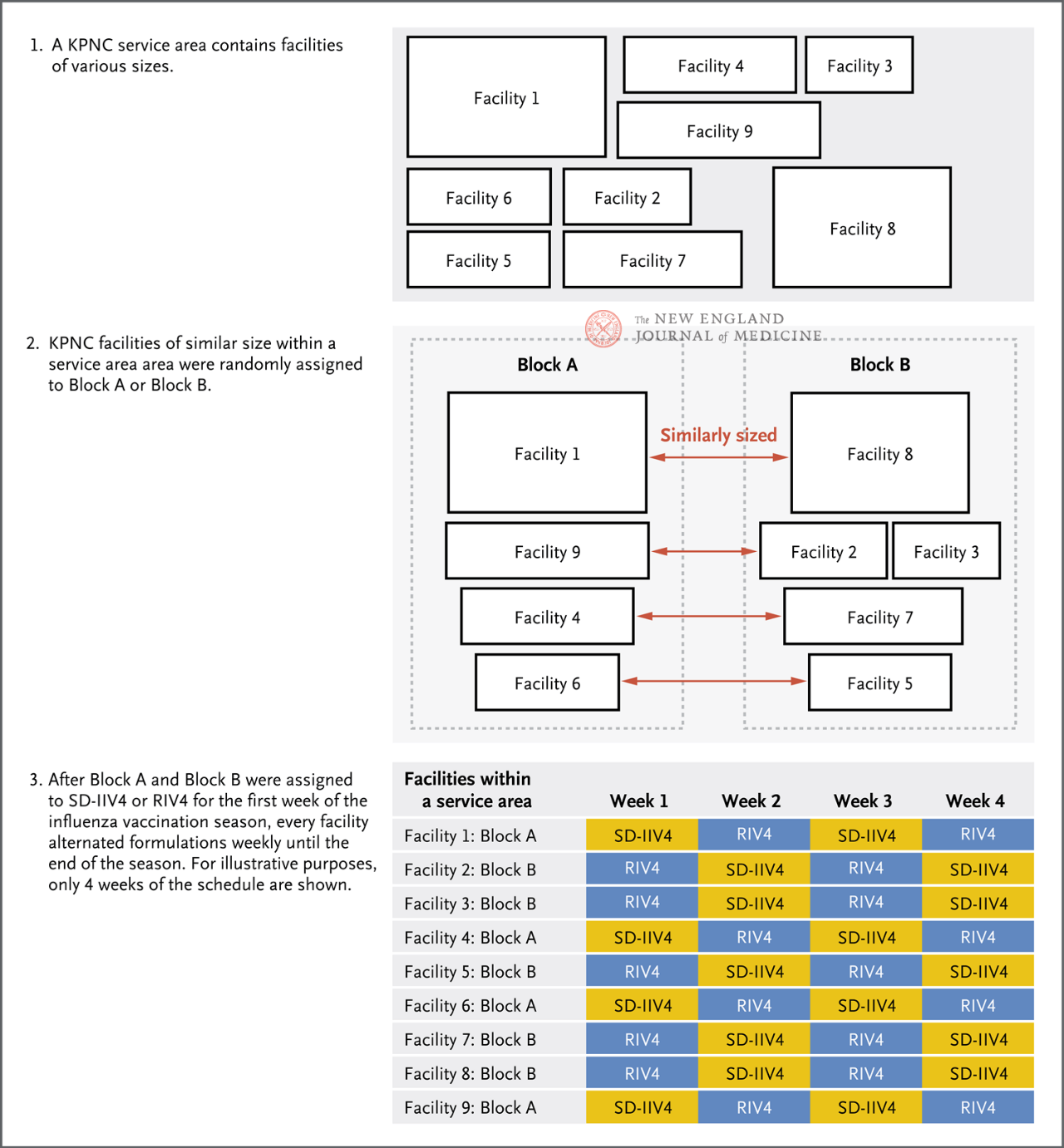
Depending on the service area and facility size of KPNC facilities, they were randomly assigned to either group A or Group B (Figure 1), where group A received RIV4 in the first week, Group B received SD-IIV4 in the first week, and then each facility received the two vaccines alternately weekly until the end of the current influenza season. The primary endpoint of the study was PCR-confirmed influenza cases, and secondary endpoints included influenza A, influenza B, and influenza-related hospitalizations. Doctors at each facility perform influenza PCR tests at their discretion, based on the clinical presentation of the patient, and obtain inpatient and outpatient diagnosis, laboratory testing, and vaccination information through electronic medical records.
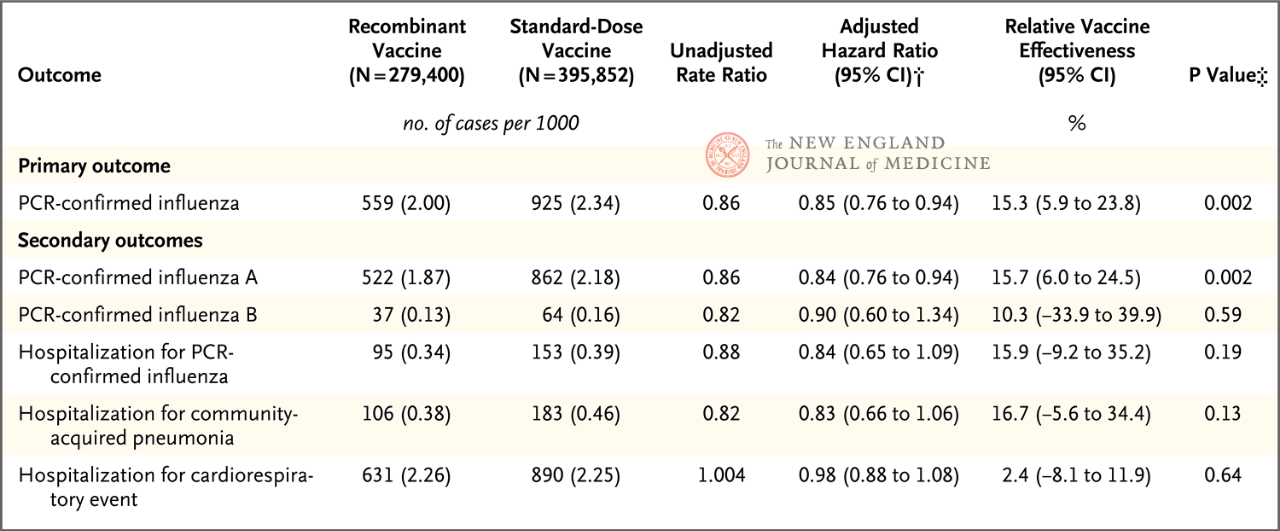
The study included adults aged 18 to 64 years, with 50 to 64 years being the primary age group analyzed. The results showed that the relative protective effect (rVE) of RIV4 compared with SD-IIV4 against PCR-confirmed influenza was 15.3% (95% CI, 5.9-23.8) in people aged 50 to 64 years. The relative protection against influenza A was 15.7% (95% CI, 6.0-24.5). No statistically significant relative protective effect was shown for influenza B or influenza-related hospitalizations. In addition, exploratory analyses showed that in people aged 18-49 years, both for influenza (rVE, 10.8%; 95% CI, 6.6-14.7) or influenza A (rVE, 10.2%; 95% CI, 1.4-18.2), RIV4 showed better protection than SD-IIV4.
A previous randomized, double-blind, positive-controlled efficacy clinical trial demonstrated that RIV4 had better protection than SD-IIV4 in people 50 years and older (rVE, 30%; 95% CI, 10~47) [3]. This study once again demonstrates through large-scale real-world data that recombinant influenza vaccines provide better protection than traditional inactivated vaccines, and complements the evidence that RIV4 also provides better protection in younger populations. The study analyzed the incidence of respiratory syncytial virus (RSV) infection in both groups (RSV infection should be comparable in both groups because influenza vaccine does not prevent RSV infection), excluded other confounding factors, and verified the robustness of the results through multiple sensitivity analyses.
The novel group randomized design method adopted in this study, especially the alternating vaccination of the experimental vaccine and the control vaccine on a weekly basis, better balanced the interfering factors between the two groups. However, due to the complexity of the design, the requirements for research execution are higher. In this study, an insufficient supply of recombinant influenza vaccine resulted in a larger number of people who should have received RIV4 receiving SD-IIV4, resulting in a larger difference in the number of participants between the two groups and a possible risk of bias. In addition, the study was originally planned to be conducted from 2018 to 2021, and the emergence of COVID-19 and its prevention and control measures have affected both the study and the intensity of the influenza epidemic, including the shortening of the 2019-2020 influenza season and the absence of the 2020-2021 influenza season. Data from only two “abnormal” flu seasons from 2018 to 2020 are available, so more research is needed to assess whether these findings hold up across multiple seasons, different circulating strains and vaccine components.
All in all, this study further proves the feasibility of recombinant genetically engineered vaccines applied in the field of influenza vaccines, and also lays a solid technical foundation for future research and development of innovative influenza vaccines. The recombinant genetic engineering vaccine technology platform does not depend on chicken embryos, and has the advantages of short production cycle and high production stability. However, compared with traditional inactivated influenza vaccines, it has no significant advantage in protection, and it is difficult to solve the immune escape phenomenon caused by highly mutated influenza viruses from the root cause. Similar to traditional influenza vaccines, strain prediction and antigen replacement are required every year.
In the face of emerging influenza variants, we should still pay attention to the development of universal influenza vaccines in the future. The development of a universal flu vaccine should gradually expand the scope of protection against virus strains, and eventually achieve effective protection against all strains in different years. Therefore, we should continue to promote the design of broad spectrum immunogen based on HA protein in the future, focus on NA, another surface protein of influenza virus, as a key vaccine target, and focus on respiratory immunization technology routes that are more advantageous in inducing multi-dimensional protective responses including local cellular immunity (such as nasal spray vaccine, inhalable dry powder vaccine, etc.). Continue to promote the research of mRNA vaccines, carrier vaccines, new adjuvants and other technical platforms, and realize the development of ideal universal influenza vaccines that “respond to all changes with no change”
Post time: Dec-16-2023