Cachexia is a systemic disease characterized by weight loss, muscle and adipose tissue atrophy, and systemic inflammation. Cachexia is one of the main complications and causes of death in cancer patients. In addition to cancer, cachexia can be caused by a variety of chronic, non-malignant diseases, including heart failure, kidney failure, chronic obstructive pulmonary disease, neurological diseases, AIDS, and rheumatoid arthritis. It is estimated that the incidence of cachexia in cancer patients can reach 25% to 70%, which seriously affects patients’ quality of life (QOL) and aggravates treatment-related toxicity.
Effective intervention of cachexia is of great significance for improving the quality of life and prognosis of cancer patients. However, despite some progress in the study of the pathophysiological mechanisms of cachexia, many drugs developed based on possible mechanisms are only partially effective or ineffective. There is currently no effective treatment approved by the U.S. Food and Drug Administration (FDA).
There are many reasons for the failure of clinical trials on cachexia, and the fundamental reason may lie in the lack of thorough understanding of the mechanism and natural course of cachexia. Recently, Professor Xiao Ruiping and researcher Hu Xinli from the College of Future Technology of Peking University jointly published an article in Nature Metabolism, revealing the important role of the lactic-GPR81 pathway in the occurrence of cancer cachexia, providing a new idea for the treatment of cachexia. We summarize this by synthesizing papers from Nat Metab, Science, Nat Rev Clin Oncol and other journals.
Weight loss is usually caused by reduced food intake and/or increased energy expenditure. Previous studies have suggested that these physiological changes in tumor-associated cachexia are driven by certain cytokines secreted by the tumor microenvironment. For example, factors such as growth differentiation factor 15 (GDF15), lipocalin-2 and insulin-like protein 3 (INSL3) can inhibit food intake by binding to appetite regulatory sites in the central nervous system, leading to anorexia in patients. IL-6, PTHrP, activin A and other factors drive weight loss and tissue atrophy by activating the catabolic pathway and increasing energy expenditure. At present, research on the mechanism of cachexia has mainly focused on these secreted proteins, and few studies have involved the association between tumor metabolites and cachexia. Professor Xiao Ruiping and researcher Hu Xinli have taken a new approach to reveal the important mechanism of tumor-related cachexia from the perspective of tumor metabolites
First, Professor Xiao Ruiping’s team screened thousands of metabolites in the blood of healthy controls and mice model of lung cancer cachexia, and found that lactic acid was the most significantly elevated metabolite in mice with cachexia. Serum lactic acid level increased with tumor growth, and showed a strong correlation with the weight change of tumor-bearing mice. Serum samples collected from lung cancer patients confirm that lactic acid also plays a key role in the progression of human cancer cachexia.
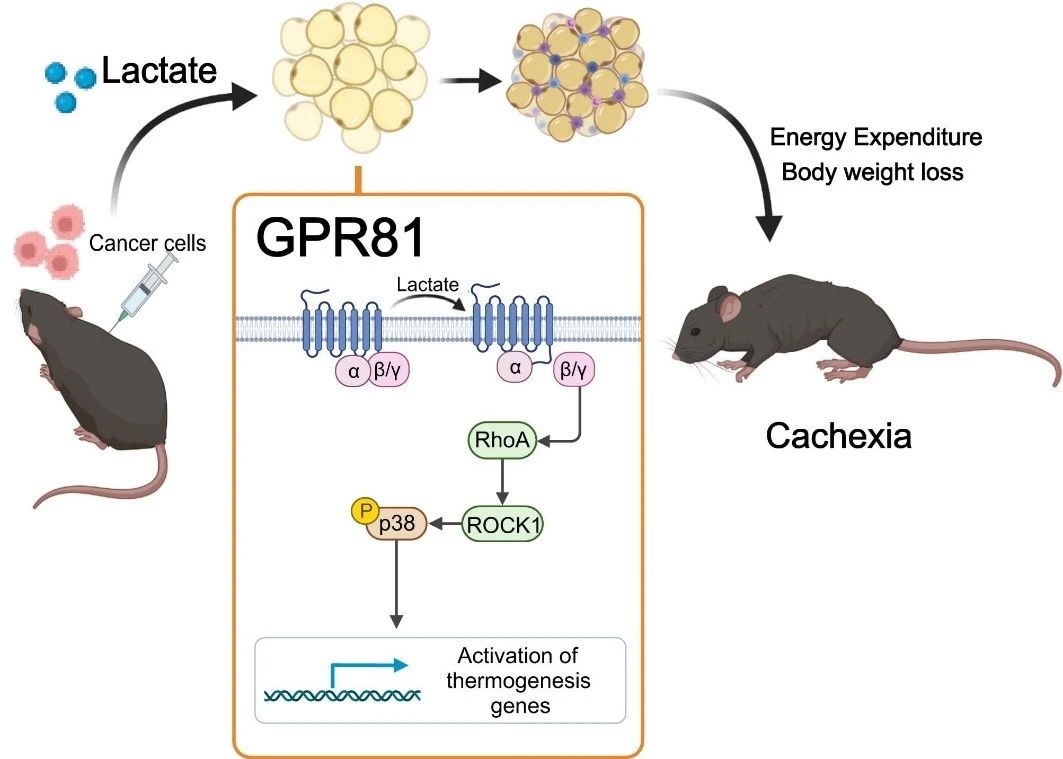
To determine whether high levels of lactic acid cause cachexia, the research team delivered lactic acid to the blood of healthy mice through an osmotic pump implanted under the skin, artificially raising serum lactic acid levels to the level of mice with cachexia. After 2 weeks, the mice developed a typical phenotype of cachexia, such as weight loss, fat and muscle tissue atrophy. These results suggest that lactate-induced fat remodeling is similar to that induced by cancer cells. Lactate is not only a characteristic metabolite of cancer cachexia, but also a key mediator of cancer-induced hypercatabolic phenotype.
Next, they found that deletion of the lactate receptor GPR81 was effective in alleviating tumor and serum lactate-induced cachexia manifestations without affecting serum lactate levels. Because GPR81 is highly expressed in adipose tissue and changes in adipose tissue earlier than skeletal muscle during the development of cachexia, the specific knockout effect of GPR81 in mouse adipose tissue is similar to that of systemic knockout, improving tumor-induced weight loss and fat and skeletal muscle consumption. This suggests that GPR81 in adipose tissue is required for the development of cancer cachexia driven by lactic acid.
Further studies confirmed that after binding to GPR81, lactic acid molecules drive fatty Browning, lipolysis and increased systemic heat production through the Gβγ-RhoA/ROCK1-p38 signaling pathway, rather than the classical PKA pathway.
Despite promising results in the pathogenesis of cancer-related cachexia, these findings have not yet translated into effective treatments, so there are currently no treatment standards for these patients, but some societies, such as ESMO and the European Society of Clinical Nutrition and Metabolism, have developed clinical guidelines. Currently, international guidelines strongly recommend promoting metabolism and reducing catabolism through approaches such as nutrition, exercise and medication
Post time: Apr-28-2024