Mixed hyperlipidemia is characterized by elevated plasma levels of low-density lipoproteins (LDL) and triglyceride-rich lipoproteins, leading to an increased risk of atherosclerotic cardiovascular disease in this patient population.
ANGPTL3 inhibits lipoprotein lipase and endosepiase, as well as liver uptake of triglyceride-rich lipoproteins. Carriers of the ANGPTL3 inactivated variant had lower levels of triglycerides, LDL cholesterol, high-density lipoprotein (HDL) cholesterol, and non-HDL cholesterol, as well as a lower risk of atherosclerotic cardiovascular disease. zodasiran is a small interfering RNA (RNAi) drug that targets ANGPTL3 expression in the liver.
Mixed hyperlipidemia refers to elevated levels of low-density lipoprotein cholesterol (LDL-C) and triglyceride-rich lipoproteins. Triglyceride-rich lipoproteins (including chylomicrons, very low density lipoproteins (VLDL), and residual cholesterol) play an important role in the development of atherosclerotic disease. There is no effective treatment for mixed hyperlipidemia.
Bates are known to reduce triglyceride (TG) levels, but the reduction is limited. At the same time, TG lowering drugs including Bates (such as eicosapentaenoic acetic acid, etc.) have no significant effect on the risk of atherosclerotic disease caused by elevated residual cholesterol levels. In addition, previous studies in patients already taking statins have shown that combination TG-lowering drugs do not reduce the risk of cardiovascular events. These factors make the treatment of mixed hyperlipidemia very difficult.
ANGPTL3 (angiopoietin-like protein 3) regulates lipids and lipoprotein metabolism, including TG and non-high-density lipoprotein cholesterol (HDL-C), by reversibly inhibiting lipoprotein lipase, endosepiase, and low-density lipoprotein (LDL) receptor-dependent hepatic lipoprotein uptake. It has been found that the ANGPTL3 inactivation variant leads to increased lipoprotein lipase and endosepiase activity, which in turn leads to low plasma lipoprotein levels in most cases, These include triglyceride-rich lipoproteins (i.e. Chylomicrons, residual cholesterol, VLDL, medium density lipoprotein [IDL]), LDL, high-density lipoprotein (HDL), lipoprotein (a), and their cholesterol components. Heterozygous people who carry this variant have an approximately 40% reduced risk of atherosclerotic disease, and no adverse clinical phenotype has been found. ANGPTL3 is expressed in the liver, and gene silencing therapies targeting its mRNA, known as small interfering RNA (siRNA) drugs, are a promising hybrid treatment for hyperlipidemia.
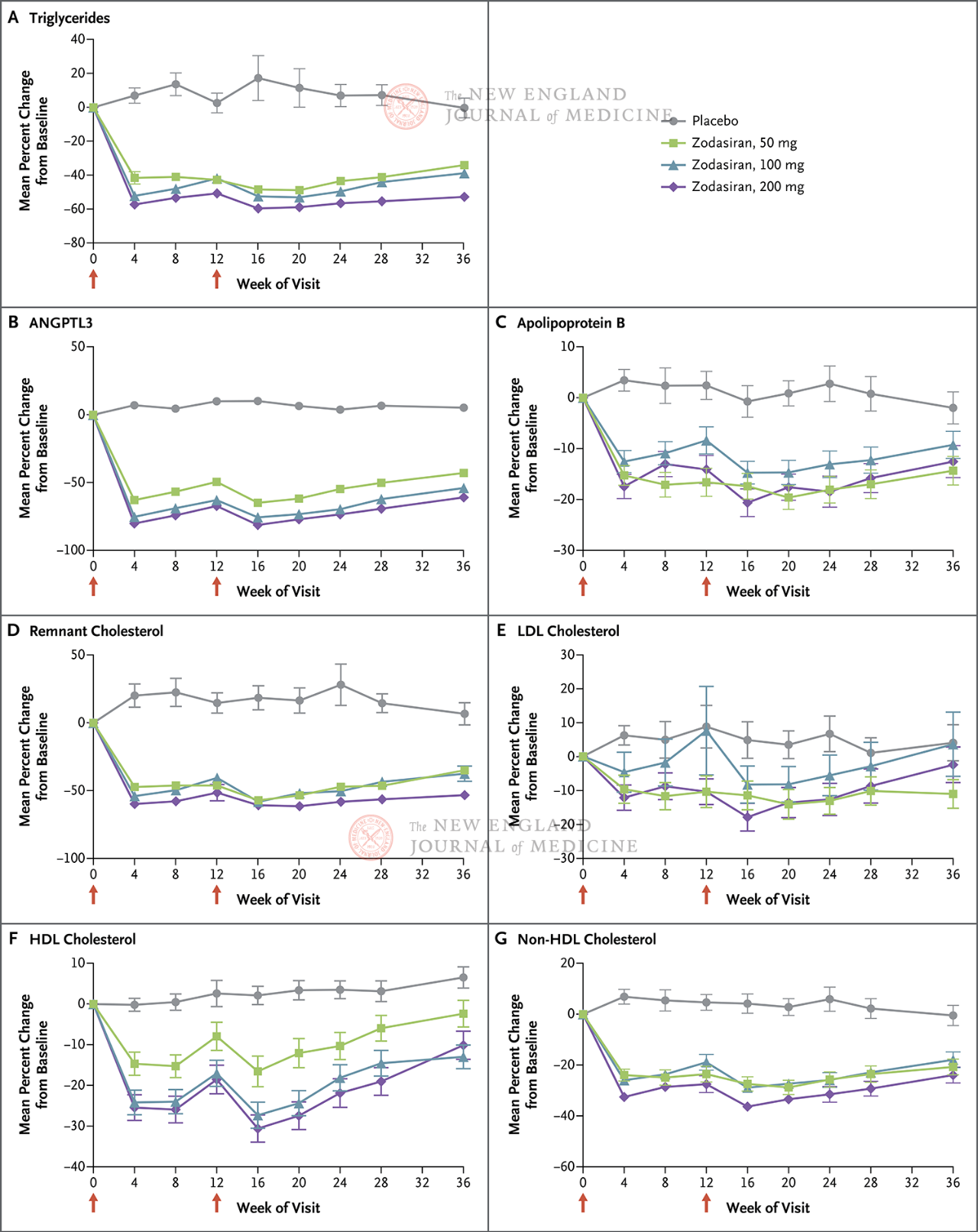
On September 12, 2024, the New England Journal of Medicine (NEJM) published a ARCHES 2 study confirming that the siRNA drug zodasiran significantly reduced TG levels in patients with mixed hyperlipidemia [1]. ARCHES-2 is a double-blind, placebo-controlled, dose-range exploration phase 2b trial. A total of 204 patients with mixed hyperlipidemia (fasting TG levels 150-499 mg/dL, LDL-C levels ³70 mg/dL or non-HDL-C levels ³100 mg/dL) were enrolled. They were divided into zodasiran 50 mg group, 100 mg group, 200 mg group and placebo control group. Patients received subcutaneous injections at week 1 and 12, and received follow-prophylaxis until week 36.
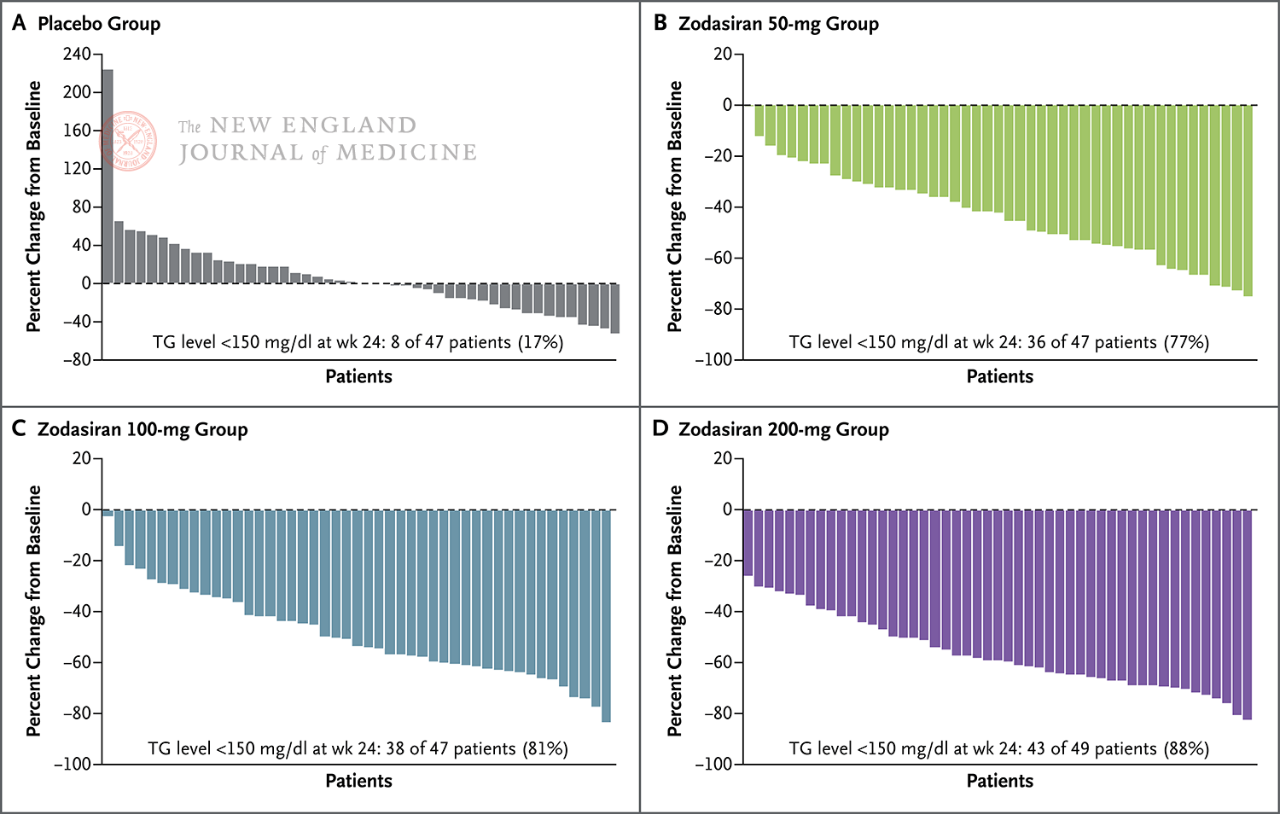
The primary endpoint was the percentage change in TG from baseline to week 24. The study found that by week 24, TG levels in the zodasiran group were significantly reduced in a dose-dependent way (TG levels in each dose group were reduced by 51, 57 and 63 percentage points, respectively, compared with those in the placebo group) (P<0.001 for all comparisons). ANGPTL3 also decreased by 54 percentage points, 70 percentage points and 74 percentage points, respectively. Non-hdl-c levels decreased by 29 percentage points, 29 percentage points, and 36 percentage points, apolipoprotein B levels decreased by 19 percentage points, 15 percentage points, and 22 percentage points, and LDL-C levels decreased by 16 percentage points, 14 percentage points, and 20 percentage points, respectively, and these results persisted until the 36th week. In week 24, zodasiran
In 88% of patients in the 200 mg group, fasting TG had fallen to the normal range.
Red arrows on days 1 and 12 indicate zodasiran or placebo administration.
Fasting TG levels decreased to normal at week 24 (150
mg/dL or less)
Each pillar represents one patient.
The study also observed that zotasiran was safe in all dose groups, with only 2 patients discontinuing the study due to adverse events (1 in the placebo group and 1 in the 100 mg zotasiran group). All serious adverse events in the zotasiran group recovered by the end of the study, and there was one death in the placebo group. The only adverse event of concern was an increase in HBA1c in the 200 mg zotasiran group compared with placebo (mean change from baseline to week 24 [±SD], 0.38±0.66% vs. -0.03±0.88% in patients with preexisting diabetes). Patients without diabetes were 0.12±0.19% vs. -0.03±0.19%).
In particular, almost all patients in the study (96%) were being treated with statins (37% of which were high-dose statins), 1% were being treated with a proprotein-converting enzyme subtilysin 9 inhibitor (PCSK9i), and 21% were being treated with fibrates. Therefore, the addition of zodasiran on the basis of the current conventional treatment regimen still achieved considerable lipid-lowering effects, which provides a new regimen for the treatment of mixed hyperlipidemia in the future.
At week 24, the maximum dose of 200 mg of zotasiran in the study reduced residual cholesterol levels by 34.4 mg/dL compared to placebo. Based on current models, this reduction is expected to reduce major cardiac adverse events by 20 percent. zodasiran has the potential to be used as a monotherapy for all lipoprotein components to reduce the risk of cardiovascular events in patients. Further research is therefore necessary to determine the potential of this drug in reducing the risk of atherosclerotic disease.
The Phase 2b, double-blind, randomized, placebo-controlled MUIR study, published simultaneously in NEJM, used another siRNA drug, plozasiran, to treat mixed hyperlipidemia [2]. plozasiran is designed to reduce the expression of APOC3, the gene encoding apolipoprotein C3 (APOC3), a regulator of TG metabolism, in the liver, thereby reducing TG and residual cholesterol levels. The reductions in TG and residual cholesterol levels observed in the study were similar to those found in the ARCHES-2 study. Therefore, it is speculated that in patients with mixed hyperlipidemia, the two drugs have similar effects in reducing the level of triglyceride-rich lipoprotein and residual cholesterol.
The results of the two siRNA studies show that this is a very promising class of drugs that will bring new options for the treatment of mixed hyperlipidemia and improve cardiovascular outcomes in patients.
Post time: Sep-15-2024