Lung transplantation is the accepted treatment for advanced lung disease. In the past few decades, lung transplantation has made remarkable progress in the screening and evaluation of transplant recipients, selection, preservation and allocation of donor lungs, surgical techniques, postoperative management, complication management, and immunosuppression.
In more than 60 years, lung transplantation has evolved from an experimental treatment to the accepted standard treatment for life-threatening lung disease. Despite common problems such as primary graft dysfunction, chronic transplant lung dysfunction (CLAD), increased risk of opportunistic infections, cancer, and chronic health problems related to immunosuppression, there is promise to improve patient survival and quality of life through the selection of the right recipient. While lung transplants are becoming more common around the world, the number of operations is still not keeping pace with the growing demand. This review focuses on the current status and recent advances in lung transplantation, as well as future opportunities for the effective implementation of this challenging but potentially life-changing therapy.
Evaluation and selection of potential recipients
Because suitable donor lungs are relatively scarce, transplant centers are ethically required to allocate donor organs to potential recipients who are most likely to gain a net benefit from transplantation. The traditional definition of such potential recipients is that they have an estimated greater than 50% risk of dying from lung disease within 2 years and a greater than 80% chance of surviving 5 years after transplantation, assuming that the transplanted lungs are fully functional. The most common indications for lung transplantation are pulmonary fibrosis, chronic obstructive pulmonary disease, pulmonary vascular disease, and cystic fibrosis. Patients are referred based on decreased lung function, decreased physical function, and disease progression despite the maximum use of medication and surgical therapies; Other disease-specific criteria are also considered. Prognostic challenges support early referral strategies that allow for better risk-benefit counseling to improve informed shared decision making and the opportunity to change potential barriers to successful transplant outcomes. The multidisciplinary team will assess the need for a lung transplant and the patient’s risk of post-transplant complications due to immunosuppressant use, such as the risk of potentially life-threatening infections. Screening for extra-pulmonary organ dysfunction, physical fitness, mental health, systemic immunity and cancer is critical. Specific assessments of coronary and cerebral arteries, kidney function, bone health, esophageal function, psychosocial capacity and social support are critical, while care is taken to maintain transparency to avoid inequities in determining suitability for transplant.
Multiple risk factors are more harmful than single risk factors. Traditional barriers to transplantation include advanced age, obesity, a history of cancer, critical illness, and concomitant systemic disease, but these factors have recently been challenged. The age of recipients is steadily increasing, and by 2021, 34% of recipients in the United States will be older than 65, indicating an increasing emphasis on biological age over chronological age. Now, in addition to the six-minute walking distance, there is often a more formal assessment of frailty, focusing on physical reserves and expected responses to stressors. Frailty is associated with poor outcomes after lung transplantation, and frailty is usually associated with body composition. Methods for calculating obesity and body composition continue to evolve, focusing less on BMI and more on fat content and muscle mass. Tools that promise to quantify faltering, oligomyosis, and resilience are being developed to better predict the ability to recover after lung transplantation. With preoperative lung rehabilitation, it is possible to modify body composition and debilitation, thereby improving outcomes.
In the case of acute critical illness, determining the extent of debilitation and ability to recover is particularly challenging. Transplants in patients receiving mechanical ventilation were previously rare, but are now becoming more common. In addition, the use of extracorporeal life support as a pre-transplant transitional treatment has increased in recent years. Advances in technology and vascular access have made it possible for conscious, carefully selected patients undergoing extracorporeal life support to participate in informed consent procedures and physical rehabilitation, and achieve outcomes after transplantation similar to those of patients who did not require extracorporeal life support before transplantation.
Concomitant systemic disease was previously considered an absolute contraindication, but its impact on post-transplant outcomes must now be specifically evaluated. Given that transplant-related immunosuppression increases the likelihood of cancer recurrence, earlier guidelines on preexisting malignancies emphasized the requirement that patients be cancer-free for five years prior to being placed on the transplant waiting list. However, as cancer therapies become more effective, it is now recommended to assess the likelihood of cancer recurrence on a patient-specific basis. Systemic autoimmune disease has traditionally been considered contraindicated, a view that is problematic because advanced lung disease tends to limit the life expectancy of such patients. The new guidelines recommend that lung transplantation should be preceded by more targeted disease assessment and treatment to reduce disease manifestations that may adversely affect outcomes, such as esophageal problems associated with scleroderma.
Circulating antibodies against specific HLA subclasses can make some potential recipients allergic to specific donor organs, resulting in longer waiting times, reduced likelihood of transplant, acute organ rejection, and elevated risk of CLAD. However, some transplants between candidate recipient antibodies and donor types have achieved similar outcomes with preoperative desensitization regimens, including plasma exchange, intravenous immunoglobulin, and anti-B cell therapy.
Selection and application of donor lung
Organ donation is an altruistic act. Obtaining donor consent and respecting their autonomy are the most important ethical factors. Donor lungs may be damaged by chest trauma, CPR, aspiration, embolism, ventilator-related injury or infection, or neurogenic injury, so many donor lungs are not suitable for transplantation. ISHLT (International Society for Heart and Lung Transplantation)
Lung Transplantation defines generally accepted donor criteria, which vary from transplant center to transplant center. In fact, very few donors meet the “ideal” criteria for lung donation (Figure 2). Increased utilization of donor lungs has been achieved through the relaxation of donor criteria (i.e., donors who do not meet conventional ideal standards), careful evaluation, active donor care, and in vitro evaluation (Figure 2). A history of active smoking by the donor is a risk factor for primary graft dysfunction in the recipient, but the risk of death from the use of such organs is limited and should be weighed against the mortality consequences of a long wait for a donor lung from a never-smoker. The use of lungs from older (older than 70 years) donors who have been rigorously selected and have no other risk factors can achieve similar recipient survival and lung function outcomes as those from younger donors.
Proper care for multiple organ donors and consideration of possible lung donation are essential to ensure that donor lungs have a high likelihood of being suitable for transplant. While few of the lungs currently provided meet the traditional definition of an ideal donor lung, relaxing the criteria beyond these traditional criteria could lead to successful utilization of organs without compromising outcomes. Standardized methods of lung preservation help protect the integrity of the organ before it is implanted in the recipient. Organs can be transported to transplant facilities under different conditions, such as cryostatic preservation or mechanical perfusion at hypothermia or normal body temperature. Lungs that are not considered suitable for immediate transplantation may be evaluated further objectively and may be treated with in vitro lung perfusion (EVLP) or preserved for longer periods of time to overcome organizational barriers to transplantation. The type of lung transplantation, procedure, and intraoperative support all depend on the patient’s needs and the surgeon’s experience and preferences. For potential lung transplant recipients whose disease deteriorates dramatically while waiting for a transplant, extracorporeal life support may be considered as a pre-transplant transitional treatment. Early postoperative complications may include bleeding, obstruction of airway or vascular anastomosis, and wound infection. Damage to the phrenic or vagus nerve in the chest can lead to other complications, affecting diaphragm function and gastric emptying, respectively. The donor lung may have early acute lung injury after implantation and reperfusion, i.e. primary graft dysfunction. It is meaningful to classify and treat the severity of primary graft dysfunction, which is associated with a high risk of early death. Because potential donor lung damage occurs within hours of the initial brain injury, lung management should include proper ventilation Settings, alveolar reexpansion, bronchoscopy and aspiration and lavage (for sampling cultures), patient fluid management, and chest position adjustment. ABO stands for blood group A, B, AB and O, CVP stands for central venous pressure, DCD stands for lung donor from cardiac death, ECMO stands for extracorporeal membrane oxygenation, EVLW stands for extravascular pulmonary water, PaO2/FiO2 stands for the ratio of arterial partial oxygen pressure to inhaled oxygen concentration, and PEEP stands for positive end-expiratory pressure. PiCCO represents the cardiac output of the pulse index waveform.
In some countries, the use of controlled donor lung (DCD) has risen to 30-40% in patients with cardiac death, and similar rates of acute organ rejection, CLAD, and survival have been achieved. Traditionally, organs from infectious virus-infected donors should be avoided for transplantation to uninfected recipients; In recent years, however, antiviral drugs that act directly against the hepatitis C virus (HCV) have enabled HCV-positive donor lungs to be safely transplanted into HCV-negative recipients. Similarly, human immunodeficiency virus (HIV) positive donor lungs can be transplanted into HIV-positive recipients, and hepatitis B virus (HBV) positive donor lungs can be transplanted into recipients who have been vaccinated against HBV and those who are immune. There have been reports of lung transplants from active or prior SARS-CoV-2 infected donors. We need more evidence to determine the safety of infecting donor lungs with infectious viruses for transplantation.
Due to the complexity of obtaining multiple organs, it is challenging to assess the quality of donor lungs. Using an in vitro lung perfusion system for evaluation allows for a more detailed assessment of donor lung function and the potential to repair it prior to use (Figure 2). Since the donor lung is highly susceptible to injury, the in vitro lung perfusion system provides a platform for the administration of specific biological therapies to repair the damaged donor lung (Figure 2). Two randomized trials have shown that in vitro normal body temperature lung perfusion of donor lungs that meet conventional criteria is safe and that the transplant team can extend preservation time in this way. Preserving donor lungs at higher hypothermia (6 to 10°C) rather than 0 to 4°C on ice has been reported to improve mitochondrial health, reduce damage, and improve lung function. For semi-selective day transplants, longer overnight preservation has been reported to achieve good post-transplant outcomes. A large non-inferior safety trial comparing preservation at 10°C with standard cryopreservation is currently underway (registration number NCT05898776 at ClinicalTrials.gov). People are increasingly promoting timely organ recovery through multi-organ donor care centers and improving organ function through organ repair centers, so that organs of better quality can be used for transplantation. The impact of these changes in the transplanting ecosystem is still being assessed.
In order to preserve controllable DCD organs, local perfusion of normal body temperature in situ via extracorporeal membrane oxygenation (ECMO) can be used to assess the function of abdominal organs and support the direct acquisition and preservation of thoracic organs, including the lungs. Experience with lung transplantation after local perfusion of normal body temperature in the chest and abdomen is limited and results are mixed. There are concerns that this procedure may cause damage to deceased donors and violate the basic ethical principles of organ harvesting; Therefore, local perfusion at normal body temperature is not yet allowed in many countries.
Cancer
The incidence of cancer in the population after lung transplantation is higher than in the general population, and the prognosis tends to be poor, accounting for 17% of deaths. Lung cancer and post-transplant lymphoproliferative disease (PTLD) are the most common causes of cancer-related death. Long-term immunosuppression, the effects of previous smoking, or the risk of underlying lung disease all lead to the risk of developing lung cancer in a single lung recipient’s own lung, but in rare cases, donor-transmitted subclinical lung cancer can also occur in transplanted lungs. Non-melanoma skin cancer is the most common cancer among transplant recipients, so regular skin cancer monitoring is essential. B-cell PTLD caused by Epstein-Barr virus is an important cause of disease and death. Although PTLD can resolve with minimal immunosuppression, B-cell targeted therapy with rituximab, systemic chemotherapy, or both is usually required.
Survival and long term outcomes
Survival after lung transplantation remains limited compared to other organ transplants, with a median of 6.7 years, and little progress has been made in patient long-term outcomes over three decades. However, many patients experienced significant improvements in quality of life, physical status, and other patient-reported outcomes; In order to conduct a more comprehensive assessment of the therapeutic effects of lung transplantation, it is necessary to pay more attention to the outcomes reported by these patients. An important unmet clinical need is to address recipient death from fatal complications of delayed graft failure or prolonged immunosuppression. For lung transplant recipients, active long-term care should be given, which requires teamwork to protect the overall health of the recipient by monitoring and maintaining graft function on the one hand, minimizing the adverse effects of immunosuppression and supporting the recipient’s physical and mental health on the other hand (Figure 1).
Future direction
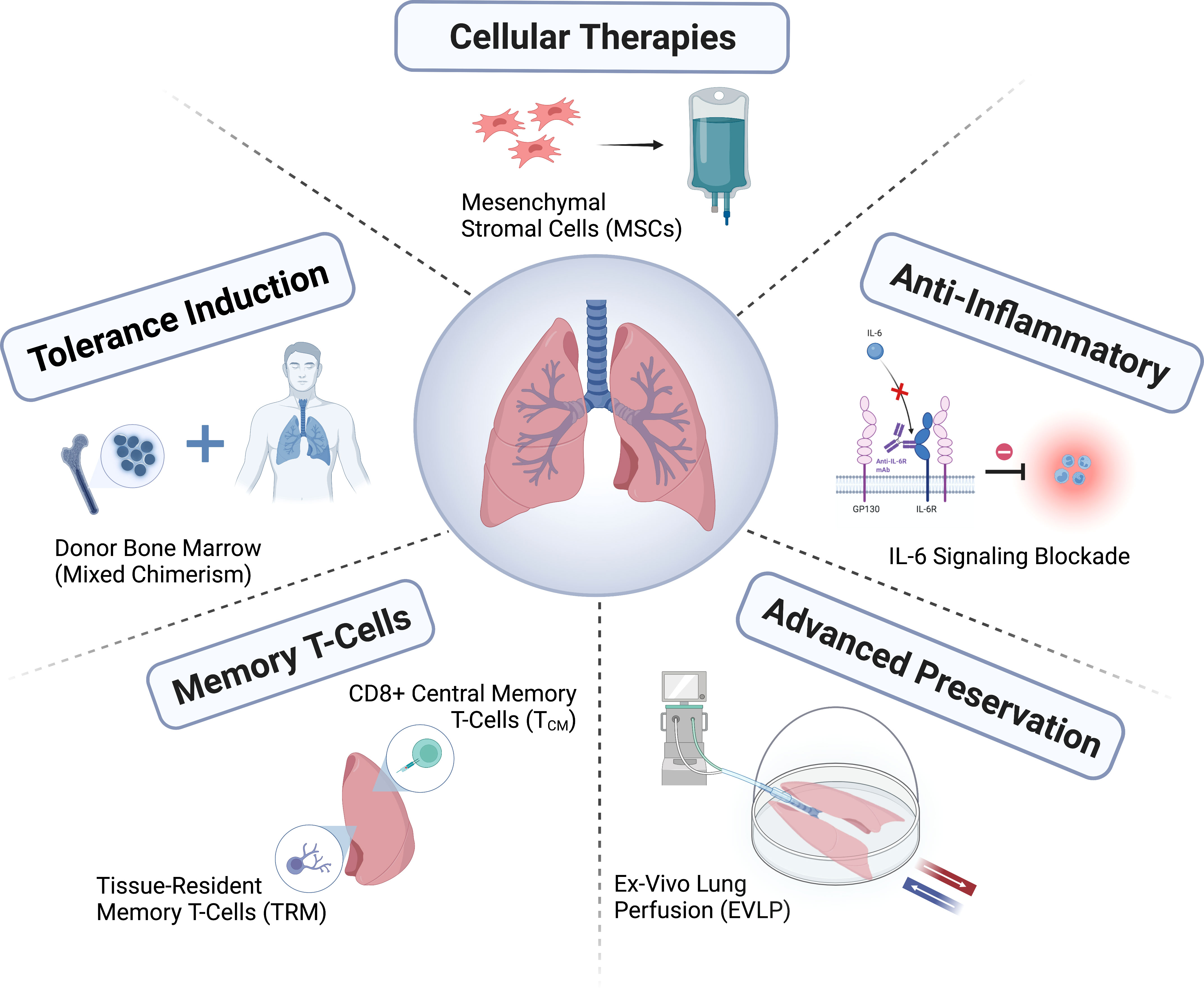
Lung transplantation is a treatment that has come a long way in a short time, but has yet to reach its full potential. The shortage of suitable donor lungs remains a major challenge, and new methods for assessing and caring for donors, treating and repairing donor lungs, and improving donor preservation are still being developed. It is necessary to improve organ allocation policies by improving the matching between donors and recipients to further increase net benefits. There is growing interest in diagnosing rejection or infection through molecular diagnostics, particularly with donor-derived free DNA, or in guiding the minimization of immunosuppression; However, the utility of these diagnostics as an adjunct to current clinical graft monitoring methods remains to be determined.
The lung transplantation field has developed through the formation of consortiums (e.g., ClinicalTrials.gov registration number NCT04787822; https://lungtransplantconsortium.org) way to work together, will help in the prevention and treatment of primary graft dysfunction, CLAD forecasting, early diagnosis and inner points (endotyping), refine syndrome, Faster progress has been made in the study of primary graft dysfunction, antibody-mediated rejection, ALAD and CLAD mechanisms. Minimizing side effects and reducing the risk of ALAD and CLAD through personalized immunosuppressive therapy, as well as defining patient-centered outcomes and incorporating them into outcome measures, will be key to improving the long-term success of lung transplantation.
Post time: Nov-23-2024