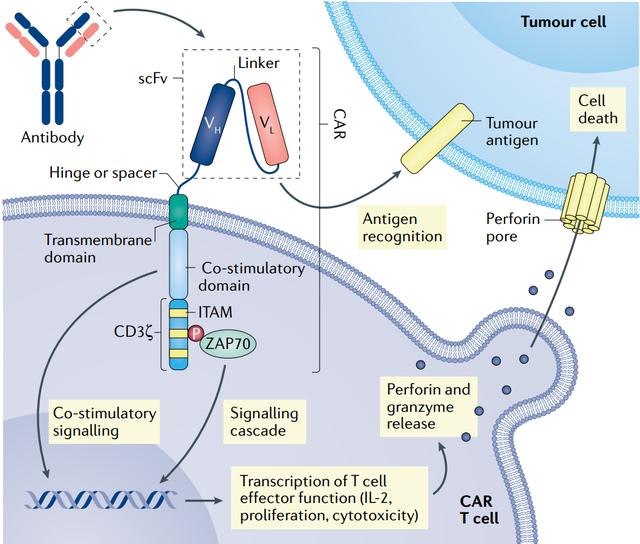
Chimeric antigen receptor (CAR) T cell therapy has become an important treatment for recurrent or refractory hematological malignancies. Currently, there are six auto-CAR T products approved for market in the United States, while there are four CAR-T products listed in China. In addition, a variety of autologous and allogeneic CAR-T products are under development. Pharmaceutical companies with these next-generation products are working to improve the efficacy and safety of existing therapies for hematological malignancies while targeting solid tumors. CAR T cells are also being developed to treat non-malignant diseases such as autoimmune diseases.
The cost of CAR T is high (at present, the cost of CAR T/ CAR in the United States is between 370,000 and 530,000 US dollars, and the cheapest CAR-T products in China are 999,000 yuan/car). Moreover, the high incidence of severe toxic reactions (especially grade 3/4 immunoeffector cell-related neurotoxic syndrome [ICANS] and cytokine release syndrome [CRS]) has become a major obstacle for low – and middle-income people to receive CAR T cell therapy.
Recently, the Indian Institute of Technology Mumbai and Mumbai Tata Memorial Hospital in cooperation to develop a new humanized CD19 CAR T product (NexCAR19), its efficacy is similar to existing products, but better safety, the most important is that the cost is only one-tenth of the United States similar products.
Like four of the six CAR T therapies approved by the U.S. Food and Drug Administration (FDA), NexCAR19 also targets CD19. However, in commercially approved products in the United States, the antibody fragment at the end of the CAR usually comes from mice, which limits its persistence because the immune system recognizes it as foreign and eventually clears it. NexCAR19 adds a human protein to the end of the mouse antibody.
Laboratory studies have shown that the antitumor activity of “humanized” Cars is comparable to that of murine-derived cars, but with lower levels of induced cytokine production. As a result, patients have a reduced risk of developing severe CRS after receiving CAR T therapy, which means that safety is improved.
To keep costs down, NexCAR19′s research team developed, tested and manufactured the product entirely in India, where labor is cheaper than in high-income countries.
To introduce CAR into T cells, researchers usually use lentiviruses, but lentiviruses are expensive. In the United States, buying enough lentiviral vectors for a 50-person trial could cost $800,000. Scientists at the NexCAR19 development company created the gene delivery vehicle themselves, dramatically reducing costs. In addition, the Indian research team has found a cheaper way to mass-produce engineered cells, avoiding the use of expensive automated machines. The NexCAR19 currently costs about $48,000 per unit, or a tenth of the cost of its U.S. counterpart. According to the head of the company that developed NexCAR19, the cost of the product is expected to be further reduced in the future.
Finally, the improved safety of this treatment compared to other FDA-approved products means that most patients do not need to recover in the intensive care unit after receiving the treatment, further reducing costs for patients.
Hasmukh Jain, a medical oncologist at the Tata Memorial Centre in Mumbai, reported a combined data analysis of Phase 1 and Phase 2 trials of NexCAR19 at the American Society of Hematology (ASH) 2023 annual meeting.
The Phase 1 trial (n=10) was a single-center trial designed to test the safety of 1×107 to 5×109 CAR T cell doses in patients with relapsed/refractory diffuse large B-cell lymphoma (r/r DLBCL), transforming follicular lymphoma (tFL), and primary mediastinal large B-cell lymphoma (PMBCL). The Phase 2 trial (n=50) was a single-arm, multicenter study that enrolled patients ≥15 years of age with r/r B-cell malignancies, including aggressive and occult B-cell lymphomas and acute lymphoblastic leukemia. Patients were given NexCAR19 two days after receiving fludarabine plus cyclophosphamide. The target dose was ≥5×107/kg CAR T cells. The primary endpoint was objective response rate (ORR), and secondary endpoints included duration of response, adverse events, progression-free survival (PFS), and overall survival (OS).
A total of 47 patients were treated with NexCAR19, 43 of whom received the target dose. A total of 33/43 (78%) patients completed the 28-day post-infusion assessment. ORR was 70% (23/33), of which 58% (19/33) achieved complete response (CR). In the lymphoma cohort, ORR was 71% (17/24) and CR was 54% (13/24). In the leukemia cohort, the CR rate was 66% (6/9, MRD-negative in 5 cases). The median follow-up time for evaluable patients was 57 days (21 to 453 days). At 3 – and 12-month follow-up, all nine patients and three-quarters of patients maintained remission.
There were no treatment-related deaths. None of the patients had any level of ICANS. 22/33 (66%) patients developed CRS (61% grade 1/2 and 6% grade 3/4). Notably, no CRS above grade 3 were present in the lymphoma cohort. Grade 3/4 cytopenia was present in all cases. The median duration of neutropenia was 7 days. At day 28, grade 3/4 neutropenia was observed in 11/33 patients (33%) and grade 3/4 thrombocytopenia was observed in 7/33 patients (21%). Only 1 patient (3%) required admission to the intensive care unit, 2 patients (6%) required vasopressor support, 18 patients (55%) received tolumab, with a median of 1 (1-4) and 5 patients (15%) received glucocorticoids. The median length of stay was 8 days (7-19 days).
This comprehensive analysis of data shows that NexCAR19 has a good efficacy and safety profile in r/r B-cell malignancies. It has no ICANS, a shorter duration of cytopenia, and a lower incidence of grade 3/4 CRS, making it one of the safest CD19 CAR T cell therapy products. The drug helps improve the ease of use of CAR T cell therapy in a variety of diseases.
At ASH 2023, another author reported on the use of medical resources in the phase 1/2 trial and the costs associated with NexCAR19 treatment. The estimated production cost of NexCAR19 at 300 patients per year in a regionally dispersed production model is approximately $15,000 per patient. At an academic hospital, the average cost of clinical management (up to the last follow-up) per patient is about $4,400 (about $4,000 for lymphoma and $5,565 for B-ALL). Only about 14 per cent of these costs are for hospital stays.
Post time: Apr-07-2024