For women of reproductive age with epilepsy, the safety of anti-seizure drugs is critical for them and their offspring, as medication is often required during pregnancy and breastfeeding to reduce the effects of seizures. Whether fetal organ development is affected by maternal antiepileptic drug treatment during pregnancy is a concern. Past studies have suggested that among traditional anti-seizure drugs, valproic acid, phenobarbital, and carbamazepine may present teratogenic risks. Among the new anti-seizure drugs, lamotrigine is considered to be relatively safe for the fetus, while topiramate may increase the risk of fetal cleft lip and palate.
Several neurodevelopmental studies have shown an association between maternal use of valproic acid during pregnancy and decreased cognitive function, autism, and attention deficit hyperactivity disorder (ADHD) in offspring. However, high-quality evidence on the relationship between maternal topiramate use during pregnancy and the neurodevelopment of offspring remains insufficient. Thankfully, a new study published last week in the New England Journal of Medicine (NEJM) brings us even more evidence
In the real world, large-scale randomized controlled trials are not possible in pregnant women with epilepsy who need antiseizure drugs to investigate the safety of the drugs. As a result, pregnancy registries, cohort studies, and case-control studies have become the more commonly used study designs. From a methodological point of view, this study is one of the high-quality studies that can be implemented at present. Its highlights are as follows: the population-based large-sample cohort study method is adopted. Although the design is retrospective, the data comes from two large national databases of the US Medicaid and Medicare systems that have been enrolled before, so the data reliability is high; The median follow-up time was 2 years, which basically met the time required for autism diagnosis, and nearly 10% (more than 400,000 cases in total) were followed for more than 8 years.
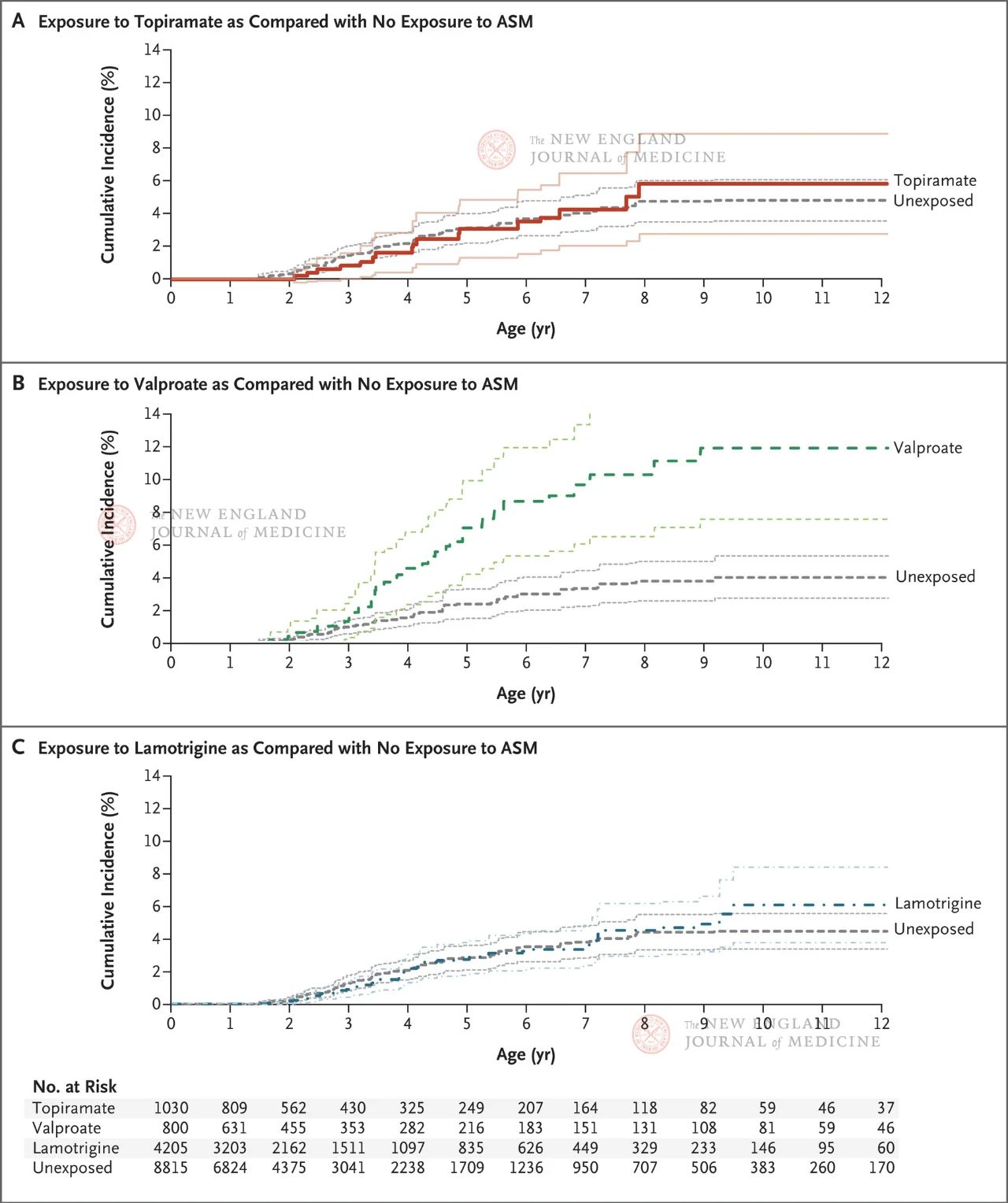
The study included more than 4 million eligible pregnant women, 28,952 of whom were diagnosed with epilepsy. Women were grouped according to whether they were taking antiepileptic drugs or different antiepileptic drugs after 19 weeks of gestation (the stage when synapses continue to form). Topiramate was in the exposed group, valproic acid was in the positive control group, and lamotrigine was in the negative control group. The unexposed control group included all pregnant women who were not taking any anti-seizure medication from 90 days before their last menstrual period to the time of delivery (also including inactive or untreated epilepsy).
The results showed that the estimated cumulative incidence of autism at age 8 was 1.89% among all progeny not exposed to any antiepileptic drugs; Among the offspring born to epileptic mothers, the cumulative incidence of autism was 4.21% (95% CI, 3.27-5.16) in children who were not exposed to antiepileptic drugs. The cumulative incidence of autism in offspring exposed to topiramate, valproate, or lamotrigine was 6.15% (95% CI, 2.98-9.13), 10.51% (95% CI, 6.78-14.24), and 4.08% (95% CI, 2.75-5.41), respectively.
Compared with fetuses not exposed to antiseizure drugs, autism risk adjusted for propensity scores was as follows: It was 0.96 (95%CI, 0.56~1.65) in the topiramate exposure group, 2.67 (95%CI, 1.69~4.20) in the valproic acid exposure group, and 1.00 (95%CI, 0.69~1.46) in the lamotrigine exposure group. In a subgroup analysis, the authors drew similar conclusions based on whether patients received monotherapy, the dose of drug therapy, and whether there was related drug exposure in early pregnancy.
The results showed that the offspring of pregnant women with epilepsy had a higher risk of autism (4.21 percent). Neither topiramate nor lamotrigine increased the risk of autism in the offspring of mothers who took antiseizure drugs during pregnancy; However, when valproic acid was taken during pregnancy, there was a dose-dependent increased risk of autism in the offspring. Although the study only focused on the incidence of autism in the offspring of pregnant women taking antiseizure drugs, and did not cover other common neurodevelopmental outcomes such as cognitive decline in the offspring and ADHD, it still reflects the relatively weak neurotoxicity of topiramate in the offspring compared with valproate.
Topiramate is generally not considered a favorable substitute for sodium valproate during pregnancy, because it can increase the risk of cleft lip and palate and small for gestational age. In addition, there are studies suggesting that topiramate may increase the risk of neurodevelopmental disorders in offspring. However, the NEJM study shows that if only considering the effect on the neurodevelopment of offspring, for pregnant women who need to use valproate for anti-epileptic seizures, it is necessary to increase the risk of neurodevelopmental disorders in offspring. Topiramate can be used as an alternative medicine. It should be noted that the proportion of Asian and other Pacific island people in the entire cohort is very low, accounting for only 1% of the whole cohort, and there may be racial differences in the adverse reactions to anti-seizure drugs, so whether the results of this study can be directly extended to Asian people (including Chinese people) needs to be confirmed by more research results of Asian people in the future.
Post time: Mar-30-2024