After entering adulthood, human hearing gradually declines. For every 10 years of age, the incidence of hearing loss almost doubles, and two-thirds of adults aged ≥ 60 suffer from some form of clinically significant hearing loss. There is a correlation between hearing loss and communication impairment, cognitive decline, dementia, increased medical costs, and other adverse health outcomes.
Everyone will gradually experience age-related hearing loss throughout their lifetime. Human auditory ability depends on whether the inner ear (cochlea) can accurately encode sound into neural signals (which are subsequently processed and decoded into meaning by the cerebral cortex). Any pathological changes in the pathway from ear to brain can have adverse effects on hearing, but age-related hearing loss involving the cochlea is the most common cause.
The characteristic of age-related hearing loss is the gradual loss of inner ear auditory hair cells responsible for encoding sound into neural signals. Unlike other cells in the body, auditory hair cells in the inner ear cannot regenerate. Under the cumulative effects of various etiologies, these cells will gradually be lost throughout a person’s life. The most important risk factors for age-related hearing loss include older age, lighter skin color (which is an indicator of cochlear pigmentation because melanin has a protective effect on the cochlea), masculinity, and noise exposure. Other risk factors include cardiovascular disease risk factors, such as diabetes, smoking and hypertension, which may lead to microvascular injury of cochlear blood vessels.
Human hearing gradually declines as they enter adulthood, especially when it comes to hearing high-frequency sounds. The incidence of clinically significant hearing loss increases with age, and for every 10 years of age, the incidence of hearing loss almost doubles. Therefore, two-thirds of adults aged ≥ 60 suffer from some form of clinically significant hearing loss.
Epidemiological studies have shown a correlation between hearing loss and communication barriers, cognitive decline, dementia, increased medical costs, and other adverse health outcomes. Over the past decade, research has particularly focused on the impact of hearing loss on cognitive decline and dementia, based on this evidence, Lancet Commission on Dementia concluded in 2020 that hearing loss in middle and old age is the largest potential modifiable risk factor for developing dementia, accounting for 8% of all dementia cases. It is speculated that the main mechanism by which hearing loss increases cognitive decline and the risk of dementia is the adverse effects of hearing loss and insufficient auditory encoding on cognitive load, brain atrophy, and social isolation.
Age related hearing loss will gradually and subtly manifest in both ears over time, without clear triggering events. It will affect the audibility and clarity of sound, as well as the daily communication experience of people. Mild hearing loss sufferers often do not realize that their hearing is declining and instead believe that their hearing difficulties are caused by external factors such as unclear speech and background noise. People with severe hearing loss will gradually notice speech clarity issues even in quiet environments, while talking in noisy environments will feel exhausted because more cognitive effort is needed to process attenuated speech signals. Usually, family members have the best understanding of the patient’s hearing difficulties.
When evaluating a patient’s hearing problems, it is important to understand that a person’s perception of hearing depends on four factors: the quality of incoming sound (such as attenuation of speech signals in rooms with background noise or echoes), the mechanical process of sound transmission through the middle ear to the cochlea (i.e. conductive hearing), the cochlea converting sound signals into neural electrical signals and transmitting them to the brain (i.e. sensorineural hearing), and the cerebral cortex decoding neural signals into meaning (i.e. central auditory processing). When a patient discovers hearing problems, the cause may be any of the four parts mentioned above, and in many cases, more than one part is already affected before the hearing problem becomes apparent.
The purpose of preliminary clinical evaluation is to evaluate whether the patient has easily treatable conductive hearing loss or other forms of hearing loss that may require further evaluation by an otolaryngologist. Conductive hearing loss that can be treated by family physicians includes otitis media and cerumen embolism, which can be determined based on medical history (such as acute onset accompanied by ear pain, and ear fullness accompanied by upper respiratory tract infection) or otoscopy examination (such as complete cerumen embolism in the ear canal). The accompanying symptoms and signs of hearing loss that require further evaluation or consultation by an otolaryngologist include ear discharge, abnormal otoscopy, persistent tinnitus, dizziness, hearing fluctuations or asymmetry, or sudden hearing loss without conductive causes (such as middle ear effusion).
Sudden sensorineural hearing loss is one of the few hearing losses that require urgent evaluation by an otolaryngologist (preferably within 3 days of onset), as early diagnosis and use of glucocorticoid intervention can improve the chances of hearing recovery. Sudden sensorineural hearing loss is relatively rare, with an annual incidence of 1/10000, most commonly in adults aged 40 or over. Compared to unilateral hearing loss caused by conductive reasons, patients with sudden sensorineural hearing loss usually report acute, painless hearing loss in one ear, resulting in almost complete inability to hear or understand others speaking.
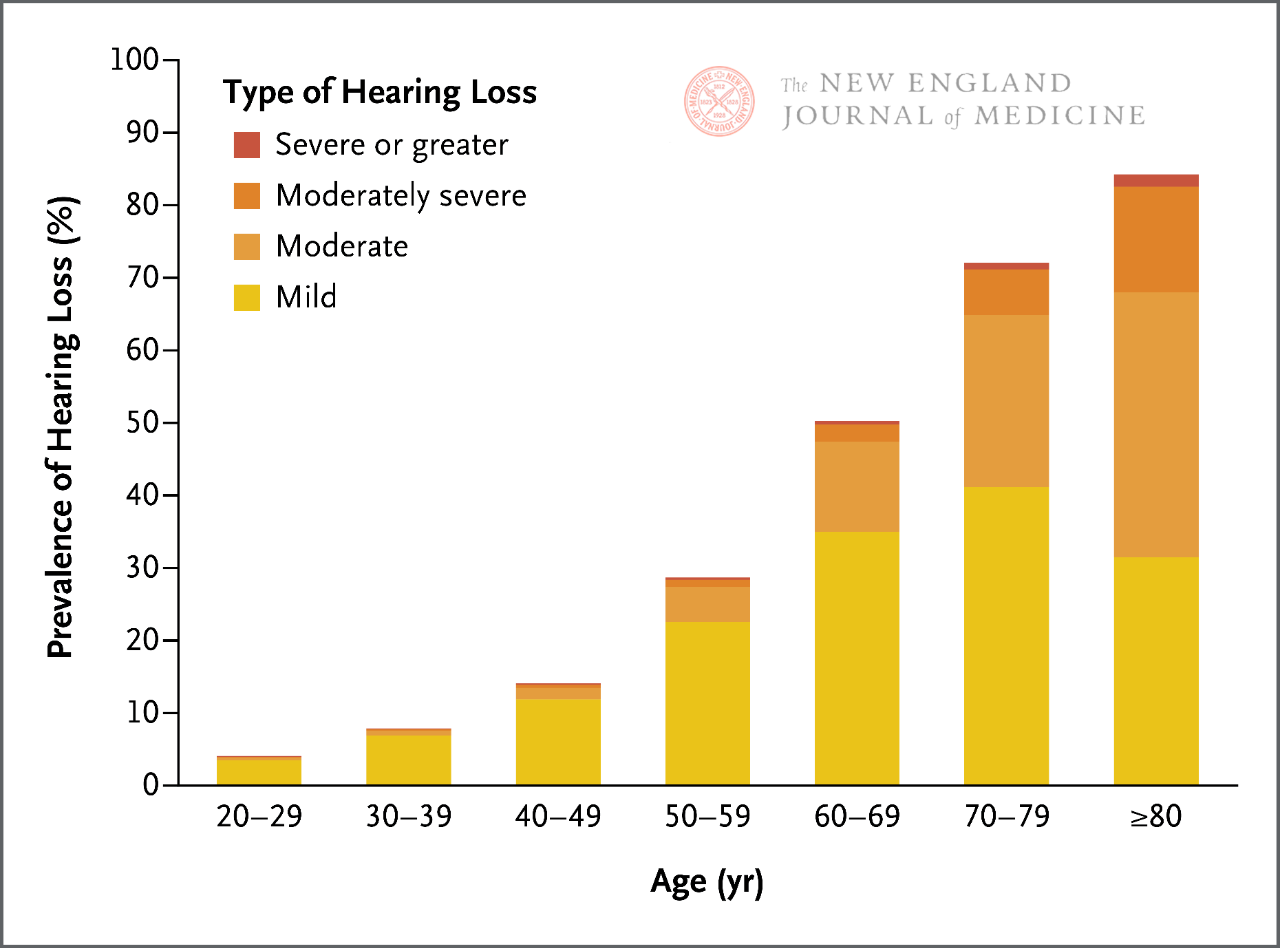
There are currently multiple bedside methods for screening for hearing loss, including whispering tests and finger twisting tests. However, the sensitivity and specificity of these testing methods vary greatly, and their effectiveness may be limited based on the likelihood of age-related hearing loss in patients. It is particularly important to note that as hearing gradually decreases throughout a person’s life (Figure 1), regardless of the screening results, it can be inferred that the patient has a certain degree of age-related hearing loss based on their age, symptoms indicating hearing loss, and no other clinical reasons.
Confirm and evaluate hearing loss and refer to an audiologist. During the hearing assessment process, the physician uses a calibrated audiometer in the soundproof room to test the patient’s hearing. Assess the minimum sound intensity (i.e. hearing threshold) that a patient can reliably detect in decibels within the range of 125-8000 Hz. A low hearing threshold indicates good hearing. In children and young adults, the hearing threshold for all frequencies is close to 0 dB, but as age increases, the hearing gradually decreases and the hearing threshold gradually increases, especially for high-frequency sounds. The World Health Organization classifies hearing based on the average threshold of a person’s hearing at the most important sound frequencies for speech (500, 1000, 2000, and 4000 Hz), known as the four frequency pure tone average [PTA4]. Clinicians or patients can understand the impact of patient hearing level on function and appropriate management strategies based on PTA4. Other tests conducted during hearing tests, such as bone conduction hearing tests and language comprehension, can also help distinguish whether the cause of hearing loss may be conductive hearing loss or central auditory processing hearing loss, and provide guidance for appropriate hearing rehabilitation plans.
The main clinical basis for addressing age-related hearing loss is to improve the accessibility of speech and other sounds in the auditory environment (such as music and sound alarms) to promote effective communication, participation in daily activities, and safety. At present, there is no restorative therapy for age-related hearing loss. The management of this disease mainly focuses on hearing protection, adopting communication strategies to optimize the quality of incoming auditory signals (beyond competing background noise), and using hearing aids and cochlear implants and other hearing technology. The usage rate of hearing aids or cochlear implants in the beneficiary population (determined by hearing) is still very low.
The focus of hearing protection strategies is to reduce noise exposure by staying away from the sound source or reducing the volume of the sound source, as well as using hearing protection devices (such as earplugs) if necessary. Communication strategies include encouraging people to have face-to-face conversations, keeping them arm’s length apart during conversations, and reducing background noise. When communicating face-to-face, the listener can receive clearer auditory signals as well as see the speaker’s facial expressions and lip movements, which helps the central nervous system decode speech signals.
Hearing aids remain the main intervention method for treating age-related hearing loss. Hearing aids can amplify sound, and more advanced hearing aids can also improve the signal-to-noise ratio of the desired target sound through directional microphones and digital signal processing, which is crucial for improving communication in noisy environments.
Non prescription hearing aids are suitable for adults with mild to moderate hearing loss, The PTA4 value is generally less than 60 dB, and this population accounts for 90% to 95% of all hearing loss patients. Compared to this, prescription hearing aids have a higher sound output level and are suitable for adults with more severe hearing loss, but can only be obtained from hearing professionals. Once the market matures, the cost of over-the-counter hearing aids is expected to be comparable to high-quality wireless earplugs. As hearing aid performance becomes a routine feature of wireless earbuds, over-the-counter hearing aids may ultimately be no different from wireless earbuds.
If the hearing loss is severe (PTA4 value generally ≥ 60 dB) and it is still difficult to understand others after using hearing aids, cochlear implant surgery may be accepted. Cochlear implants are neural prosthetic devices that encode sound and directly stimulate the cochlear nerves. It is implanted by an otolaryngologist during outpatient surgery, which takes about 2 hours. After implantation, patients need 6-12 months to adapt to hearing achieved through cochlear implants and perceive neural electrical stimulation as meaningful language and sound.
Post time: May-25-2024