Nowadays, Nonalcoholic fatty liver disease (NAFLD) has become the main cause of chronic liver disease in China and even in the world. The disease spectrum includes simple hepatic steatohepatitis, nonalcoholic steatohepatitis (NASH) and related cirrhosis and liver cancer. NASH is characterized by excess fat accumulation in hepatocytes and induced cellular damage and inflammation, with or without hepatic fibrosis. The severity of liver fibrosis in NASH patients is closely associated with poor liver prognosis (cirrhosis and its complications and hepatocellular carcinoma), cardiovascular events, extrahepatic malignancies, and all-cause death. NASH can adversely affect patients’ quality of life; however, no drugs or therapies have been approved to treat NASH.
A recent study (ENLIVEN) published in the New England Journal of Medicine (NEJM) showed that pegozafermin improved both liver fibrosis and liver inflammation in biopsy-confirmed non-cirrhotic NASH patients.
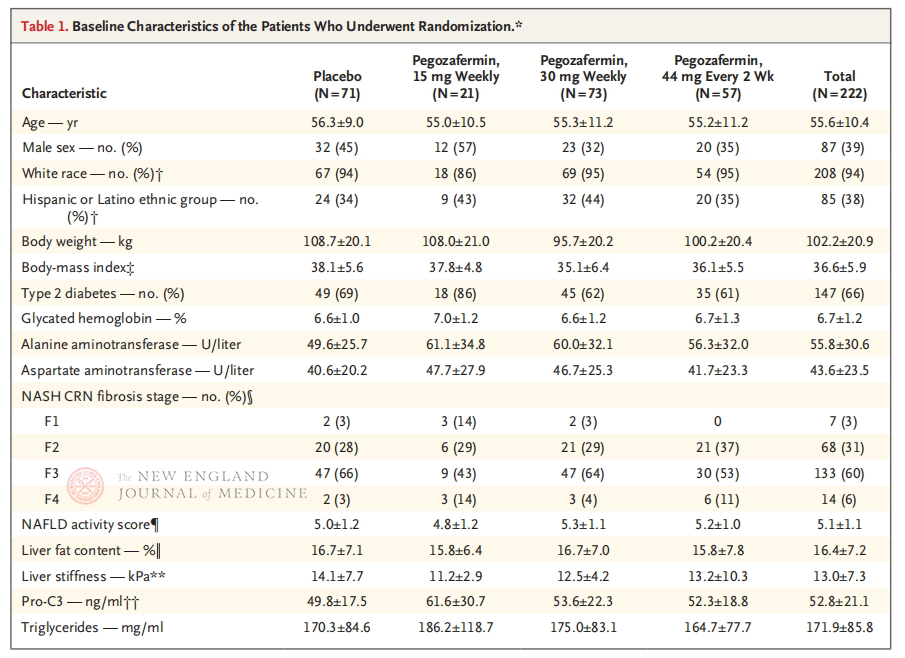
The multicenter, randomized, double-blind, placebo-controlled Phase 2b clinical trial, conducted by Professor Rohit Loomba and his clinical team at the University of California, San Diego School of Medicine, enrolled 222 patients with biopsie-confirmed stage F2-3 NASH between September 28, 2021 and August 15, 2022. They were randomly assigned to pegozafermin (subcutaneous injection, 15 mg or 30 mg once a week, or 44 mg once every 2 weeks) or placebo (once a week or once every 2 weeks). Primary endpoints included ≥ stage 1 improvement in fibrosis and no progression of NASH. NASH resolved without fibrotic progression. The study also conducted a safety assessment.
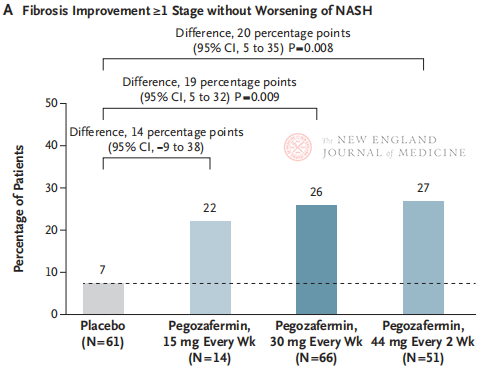
After 24 weeks of treatment, the proportion of patients with ≥ stage 1 improvement in fibrosis and no worsening of NASH, and the proportion of patients with regression of NASH and no worsening of fibrosis were significantly higher in the three Pegozafermin dose groups than in the placebo group, with more significant differences in patients treated with 44 mg once every two weeks or 30 mg once every week. In terms of safety, pegozafermin was similar to placebo. The most common adverse events associated with pegozafermin treatment were nausea, diarrhea, and erythema at the injection site. In this phase 2b trial, preliminary results suggest that treatment with pegozafermin improves liver fibrosis.
pegozafermin, used in this study, is a long-acting glycolated analogue of human fibroblast growth factor 21 (FGF21). FGF21 is an endogenous metabolic hormone secreted by the liver, which plays a role in regulating lipid and glucose metabolism. Previous studies have shown that FGF21 has therapeutic effects on NASH patients by increasing liver insulin sensitivity, stimulating fatty acid oxidation, and inhibiting lipogenesis. However, the short half-life of natural FGF21 (about 2 hours) limits its use in the clinical treatment of NASH. pegozafermin utilizes glycosylated pegylation technology to extend the half-life of natural FGF21 and optimize its biological activity.
In addition to the positive results in this Phase 2b clinical trial, another recent study published in Nature Medicine (ENTRIGUE) showed that pegozafermin also significantly reduced triglycerides, non-HDL cholesterol, apolipoprotein B, and hepatic steatosis in patients with severe hypertriglyceridemia, which may have a positive impact on reducing the risk of cardiovascular events in patients with NASH.
These studies suggest that pegozafermin, as an endogenous metabolic hormone, can provide multiple metabolic benefits to patients with NASH, especially because NASH may be renamed metabolically associated fatty liver disease in the future. These results make it a very important potential drug for the treatment of NASH. At the same time, these positive study results will support pegozafermin into phase 3 clinical trials.
Although both biweekly 44 mg or weekly 30 mg pegozafermin treatment achieved the histological primary endpoint of the trial, the duration of treatment in this study was only 24 weeks, and the compliance rate in the placebo group was only 7%, which was significantly lower than the results of previous clinical studies lasting 48 weeks. Are the differences and security the same? Given the heterogeneity of NASH, larger, multi-center, international clinical trials are needed in the future to include larger patient populations and extend treatment duration to better evaluate the efficacy and safety of the drug.
Post time: Sep-16-2023