Cachexia is a systemic disease characterized by weight loss, muscle and adipose tissue atrophy, and systemic inflammation. Cachexia is one of the main complications and causes of death in cancer patients. It is estimated that the incidence of cachexia in cancer patients can reach 25% to 70%, and about 9 million people worldwide suffer from cachexia each year, 80% of whom are expected to die within one year of diagnosis. In addition, cachexia significantly affects patient quality of life (QOL) and exacerbates treatment-related toxicity.
Effective intervention of cachexia is of great significance for improving the quality of life and prognosis of cancer patients. However, despite some progress in the study of the pathophysiological mechanisms of cachexia, many drugs developed based on possible mechanisms are only partially effective or ineffective. There is currently no effective treatment approved by the U.S. Food and Drug Administration (FDA).
Cachexia (wasting syndrome) is very common in patients with many types of cancer, often resulting in weight loss, muscle wasting, reduced quality of life, impaired function, and shortened survival. According to internationally agreed standards, this multifactorial syndrome is defined as a body mass index (BMI, weight [kg] divided by height [m] squared) of less than 20 or, in patients with sarcopenia, a weight loss of more than 5% in six months, or a weight loss of more than 2%. Currently, no drugs have been approved in the United States and Europe specifically for the treatment of cancer cachexia, resulting in limited treatment options.
Recent guidelines recommending low-dose olanzapine to improve appetite and weight in patients with advanced cancer are largely based on the results of a single-center study. In addition to this, short-term use of progesterone analogues or glucocorticoids may confer limited benefits, but there is a risk of adverse side effects (such as progesterone use associated with thromboembolic events). Clinical trials of other drugs have failed to show efficacy sufficient to win regulatory approval. Although anamorine (an oral version of growth hormone releasing peptides) has been approved in Japan for the treatment of cancer cachexia, the drug only increased body composition to a certain extent, did not improve grip strength, and was ultimately not approved by the US Food and Drug Administration (FDA). There is an urgent need for safe, effective and targeted treatments for cancer cachexia.
Growth differentiation factor 15 (GDF-15) is a stress-induced cytokine that binds to the glia-derived neurotrophic factor family receptor alpha-like protein (GFRAL) in the posterior brain. The GDF-15-GFRAL pathway has been identified as a major regulator of anorexia and weight regulation, and plays a role in the pathogenesis of cachexia. In animal models, GDF-15 can induce cachexia, and inhibition of GDF-15 can alleviate this symptom. In addition, elevated levels of GDF-15 in cancer patients are associated with decreased body weight and skeletal muscle mass, decreased strength, and shortened survival, underscoring the value of GDF-15 as a potential therapeutic target.
ponsegromab (PF-06946860) is a highly selective humanized monoclonal antibody capable of binding to circulating GDF-15, thereby inhibiting its interaction with the GFRAL receptor. In a small open-label phase 1b trial, 10 patients with cancer cachexia and elevated circulating GDF-15 levels were treated with ponsegromab and showed improvements in weight, appetite, and physical activity, while serum GDF-15 levels were inhibited and adverse events were low. Based on this, we conducted a Phase 2 clinical trial to evaluate the safety and efficacy of ponsegromab in patients with cancer cachexia with elevated circulating GDF-15 levels, compared with placebo, to test the hypothesis that GDF-15 is the primary pathogenesis of the disease.
The study included adult patients with cachexia associated with cancer (non-small cell lung cancer, pancreatic cancer, or colorectal cancer) with a serum GDF-15 level of at least 1500 pg/ml, an Eastern Tumor Consortium (ECOG) fitness status score of ≤3, and a life expectancy of at least 4 months.
Enrolled patients were randomly assigned to receive 3 doses of ponsegromab 100 mg, 200 mg, or 400 mg, or placebo, subcutaneously every 4 weeks in a ratio of 1:1:1. The primary endpoint was change in body weight relative to baseline at 12 weeks. The key secondary endpoint was the change from baseline in the anorexia cachexia Sub-Scale (FAACT-ACS) score, an assessment of therapeutic function for anorexia cachexia. Other secondary endpoints included cancer-associated cachexia symptom diary scores, baseline changes in physical activity and gait endpoints measured using wearable digital health devices. Minimum wear time requirements are specified in advance. The safety assessment included the number of adverse events during treatment, laboratory test results, vital signs, and electrocardiograms. Exploratory endpoints included baseline changes in lumbar skeletal muscle index (skeletal muscle area divided by height squared) associated with systemic skeletal muscle.
A total of 187 patients were randomly assigned to receive ponsegromab 100 mg(46 patients), 200 mg(46 patients), 400 mg(50 patients), or placebo (45 patients). Seventy-four (40 percent) had non-small cell lung cancer, 59 (32 percent) had pancreatic cancer, and 54 (29 percent) had colorectal cancer.
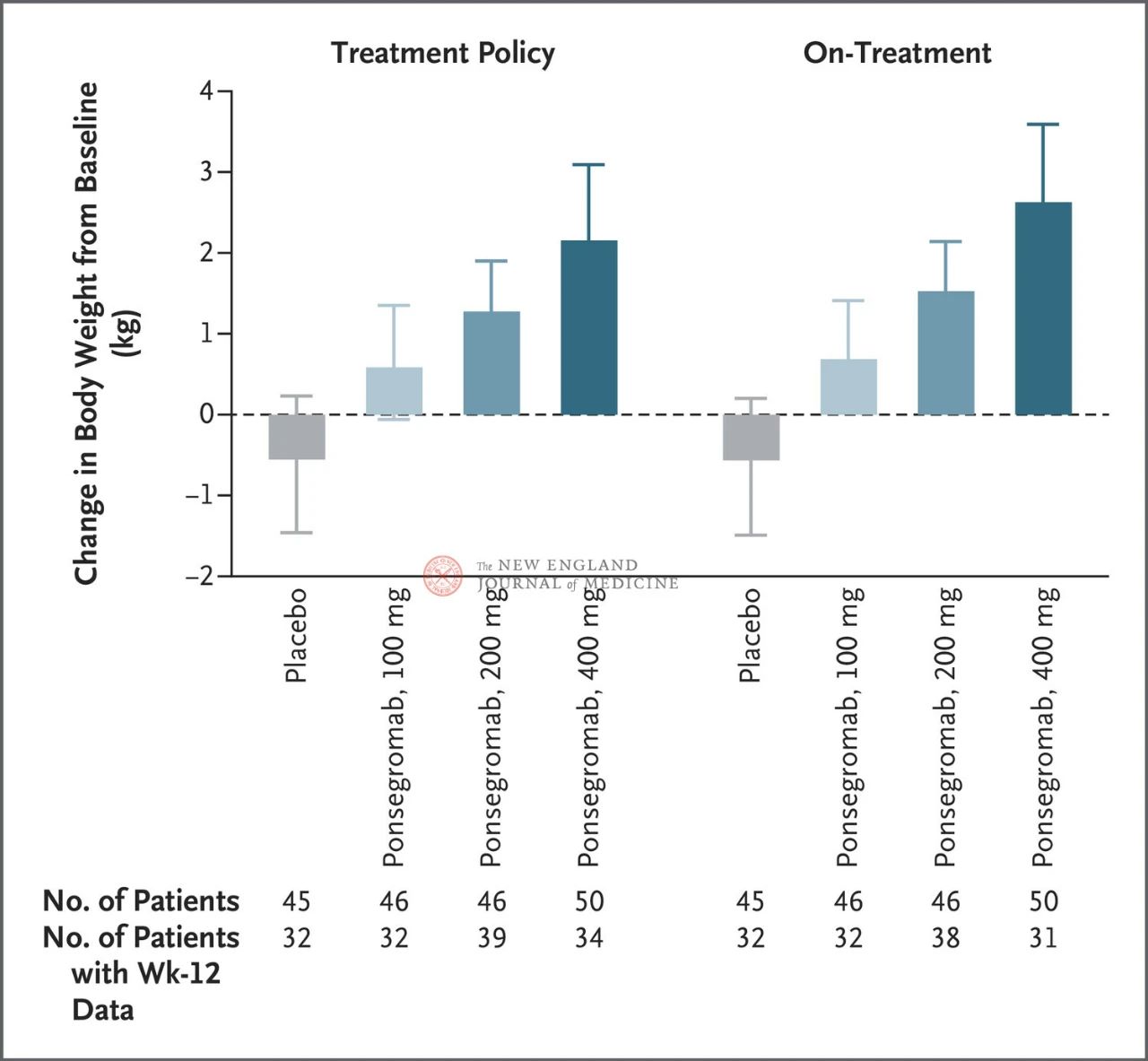
The differences between the 100 mg, 200 mg, and 400 mg groups and placebo were 1.22 kg, 1.92 kg, and 2.81 kg, respectively
The figure shows the primary endpoint (change in body weight from baseline to 12 weeks) for patients with cancer cachexia in the ponsegromab and placebo groups. After adjusting for the competing risk of death and other concurrent events, such as treatment interruption, the primary endpoint was analyzed by a stratified Emax model using week 12 results from a Bayesian joint longitudinal analysis (left). The primary endpoints were also analyzed in a similar manner, using estimated targets for actual treatment, where observations after all concurrent events were truncated (right figure). Confidence intervals (indicated in article
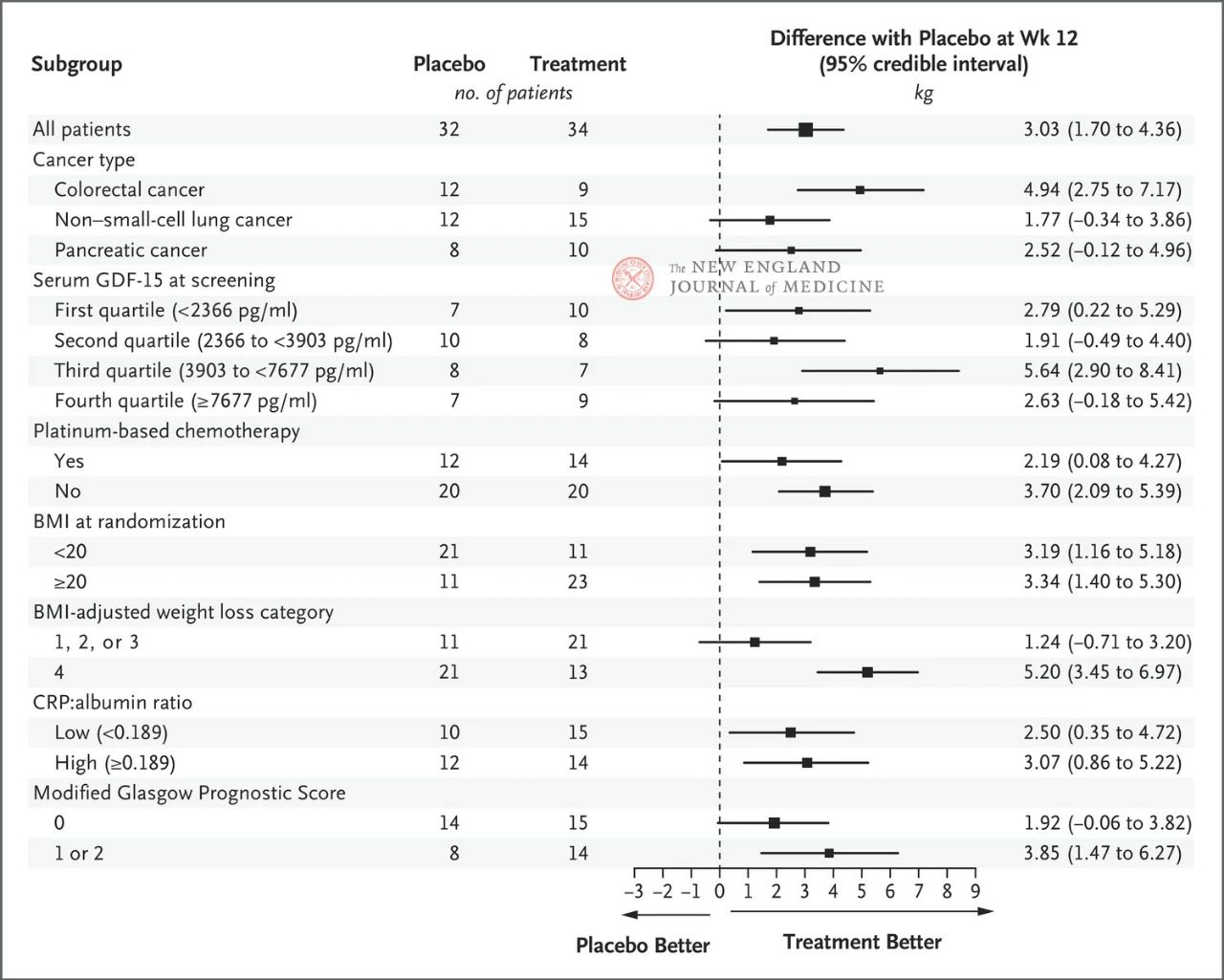
The effect of 400 mg ponsegromab on body weight was consistent across major preset subgroups, including cancer type, serum GDF-15 level quartile, platinum-based chemotherapy exposure, BMI, and baseline systemic inflammation. Weight change was consistent with GDF-15 inhibition at 12 weeks.
The selection of key subgroups was based on a post-hoc Bayesian joint longitudinal analysis, which was conducted after adjusting for the competitive risk of death based on the estimated target of the treatment strategy. Confidence intervals should not be used as a substitute for hypothesis testing without multiple adjustments. BMI represents body mass index, CRP represents C-reactive protein, and GDF-15 represents growth differentiation factor 15.
At baseline, a higher proportion of patients in the ponsegromab 200 mg group reported no decrease in appetite; Compared with placebo, patients in the ponsegromab 100 mg and 400 mg groups reported an improvement in appetite from baseline at 12 weeks, with an increase in FAACT-ACS scores of 4.12 and 4.5077, respectively. There was no significant difference in the FAACT-ACS scores between the 200 mg group and the placebo group.
Due to pre-specified wear time requirements and device issues, 59 and 68 patients, respectively, provided data on changes in physical activity and gait endpoints relative to baseline. Among these patients, compared to the placebo group, patients in the 400 mg group had an increase in overall activity at 12 weeks, with an increase of 72 minutes of non-sedentary physical activity per day. In addition, the 400 mg group also had an increase in lumbar skeletal muscle index at week 12.
The incidence of adverse events was 70% in the ponsegromab group, compared with 80% in the placebo group, and occurred in 90% of patients receiving systemic anticancer therapy simultaneously. The incidence of nausea and vomiting was lower in ponsegromab group.
Post time: Oct-05-2024